Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Groundbreaking Chinese CAR-T Research: CD5 CAR-T Therapy Brings New Hope for Relapsed/Refractory T-cell Acute Lymphoblastic Leukemia (T-ALL)
Groundbreaking Chinese CAR-T Research: CD5 CAR-T Therapy Brings New Hope for Relapsed/Refractory T-cell Acute Lymphoblastic Leukemia (T-ALL)

T-ALL
Relapsed or refractory acute T-cell lymphoblastic leukemia (r/r T-ALL) has long lacked effective treatment options, with a poor prognosis. Although CD7 CAR-T therapy has shown some efficacy, many patients experience relapse due to CD7 antigen loss.
#CAR-T #TALL #ALL #CARTtherapy #leukemia #NatureMedicine #Tcell #CD5
Breakthrough Research: CD5 CAR-T Therapy Published in Nature Medicine
To address this challenge, a team of Chinese medical professors published groundbreaking research in Nature Medicine titled “Allogeneic CD5-specific CAR-T therapy for relapsed/refractory T-ALL: a phase 1 trial.” This study explores the application of donor-derived CD5 CAR-T therapy in r/r T-ALL, offering a new possibility for the treatment of T-cell hematologic malignancies.
Study Design: Exploring the Safety and Efficacy of CAR-T Therapy
This study focused on a 21-day dose-limiting toxicity (DLT) observation period and adverse reactions within 30 days, preliminarily confirming the short-term safety of CD5 CAR-T therapy. Excitingly, early results showed complete remission in all enrolled patients, demonstrating significant short-term efficacy. Despite this positive progress, the research team also observed that, in long-term follow-up, patients who did not receive bridging transplantation faced a risk of functional deficits due to T-cell insufficiency. Addressing this challenge will be a key focus for the next stage of optimization.
Future Outlook: Exceptional Progress by the Chinese Team Brings New Hope to Patients
In future research, the Chinese medical and scientific team will continue efforts to enhance the safety of CAR-T therapy, particularly by adding “switch” components to allow flexible activation or deactivation of the therapy in patients, aiming for durable efficacy. If successful, this optimization could greatly expand the potential of CAR-T therapy in treating T-cell malignancies.
This research not only brings hope to patients with relapsed/refractory T-ALL but also signifies outstanding progress by the Chinese medical and research team in innovative therapies. The work of Professor Pan and his team highlights the leading position of China’s CAR-T therapy in global cancer immunotherapy and holds promise for bringing new life-saving options to more patients with T-cell hematologic malignancies.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#TALLTreatment #Immunotherapy #CancerResearch #Breakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breakthrough in China’s CAR-T Therapy for Treating Relapsed B-ALL: Donor-Derived CD19 CAR-T Shows Long-Term Survival Advantage
**Breakthrough in China’s CAR-T Therapy for Treating Relapsed B-ALL: Donor-Derived CD19 CAR-T Shows Long-Term Survival Advantage**

B-ALL
#CAR_TTherapy #B_ALL #DonorCAR_T #CAR_T #ALL #ChinaCART #CD19 #alloHSCT
In recent years, China has made significant strides in CAR-T cell therapy, particularly in treating B-cell acute lymphoblastic leukemia (B-ALL). Donor-derived CAR-T therapy has shown promising efficacy for B-ALL patients who relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT), bringing new hope for long-term survival.
A study jointly published by the Chinese Academy of Medical Sciences and Chinese medical teams in the *Journal of Hematology & Oncology*, titled “Long-term survival with donor CD19 CAR-T cell treatment for relapsed patients after allogeneic hematopoietic stem cell transplantation,” indicates that patients treated with donor CD19 CAR-T cells achieved complete remission (CR) without requiring a second transplant, with a significant increase in long-term survival rates. This study followed 32 B-ALL patients who relapsed post-allo-HSCT. The median patient age was 24, and after receiving donor CD19 CAR-T therapy, they achieved complete remission or partial recovery in peripheral blood (CRi), with a median follow-up of 42 months.
Results showed that patients treated with donor CD19 CAR-T had a 2-year overall survival (OS) rate of 56.25% and an event-free survival (EFS) rate of 50.0%. The 5-year OS and EFS reached 53.13% and 46.88%, respectively, with no new long-term adverse events. These findings suggest that donor CAR-T cells are not only effective but also have long-term safety advantages over second transplantation or traditional donor lymphocyte infusion, offering a more promising treatment option for relapsed B-ALL patients.
While these results are encouraging, further development of early detection methods is needed to identify or prevent relapse at an earlier stage. Early relapse with donor CAR-T, especially within the first six months post-treatment, remains a challenge, requiring additional multi-center, prospective studies for validation. Overall, this research provides robust clinical data supporting donor CAR-T therapy for relapsed B-ALL patients post-transplant, potentially establishing a benchmark for CAR-T therapies in China on a global scale.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#ChinaMedicalBreakthrough #CancerResearch #Hematology #Oncology #LongTermSurvival #StemCellTransplantation #RelapsedLeukemia #ChineseMedicalResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s CAR-T Therapy: A Beacon of Hope for Children with Acute Lymphoblastic Leukemia (ALL)
China’s CAR-T Therapy: A Beacon of Hope for Children with Acute Lymphoblastic Leukemia (ALL)

#CARTTherapy #Leukemia #ChildhoodCancer #ALL #CART #AcuteLymphoblasticLeukemia #Patientstory
China’s CAR-T cell therapy is emerging as a beacon of hope in the fight against cancer, bringing unprecedented treatment possibilities. As a precise cellular immunotherapy, CAR-T therapy enhances the cancer-fighting abilities of T-cells through genetic editing technology, offering breakthroughs for patients with cancers resistant to traditional treatments, especially in blood cancers like leukemia.
From Diagnosis to Treatment: Strength and Struggle
Shan is a young child in China who has endured long-term treatment for leukemia. Diagnosed with acute lymphoblastic leukemia (ALL) in 2019, this news brought immense suffering to her and her family, as she was only five years old. Although ALL generally responds well to chemotherapy, Shan’s cancer cells persisted stubbornly after several rounds of chemotherapy, failing to reach full remission. Her doctors recommended a bone marrow transplant, but due to physical and family constraints, she had to opt for conservative treatment. Nevertheless, Shan did not give up her desire to live, showing remarkable resilience over five years.
A New Hope Through CAR-T Therapy
In 2023, Shan’s condition worsened as her anemia grew severe, with her hemoglobin levels dropping to a critical point. Faced with despair, her mother reached out to our team at Advanced Medicine in China, where experts quickly admitted her to the hospital. After a comprehensive evaluation, our hematology-oncology specialists decided to proceed with CAR-T therapy for her.
Following a thorough process of gene modification and the infusion of CAR-T cells, Shan’s condition improved rapidly. In the early stages of treatment, her complexion brightened, her energy levels noticeably improved, and subsequent tests showed that her tumor cells had completely disappeared, achieving clinical remission. Shan was finally free from the shadow of cancer, gradually regaining the joy and health of childhood.
A Medical Breakthrough: New Hope for Children with Leukemia
Shan’s success story signifies the maturation of CAR-T therapy in China, showcasing its powerful potential, particularly in treating refractory and relapsed childhood acute leukemia. In recent years, Chinese medical teams have actively adopted CAR-T technology in treating pediatric blood cancers, achieving remarkable long-term remission for many children. As the country with the highest number of CAR-T therapy applications globally, China’s hospital teams possess extensive experience and advanced technological capabilities, offering high-quality treatment to more children suffering from relapsed and refractory blood diseases.
Moving Forward: A Promising Future for Cellular Immunotherapy
With the continued development of CAR-T therapy in China, more patients with challenging cancers stand to benefit. Shan’s recovery is not only a family’s joy but also a testament to China’s medical progress. CAR-T therapy is ushering in a new era of cellular immunotherapy, giving more patients the possibility of life extension and recovery.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+861371795907
Email: doctor.huang@globecancer.com
#CancerResearch #MedicalBreakthrough #Immunotherapy #ChinaMedicine #Oncology #CancerHope #LeukemiaAwareness #CancerTreatment
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Significant Progress in CAR-T Therapy in China: Long-Term Effects of Combined CD19 and CD22 Treatment for Acute B-Lymphoblastic Leukemia
### Significant Progress in CAR-T Therapy in China: Long-Term Effects of Combined CD19 and CD22 Treatment for Acute B-Lymphoblastic Leukemia

Leukemia
#ALL #CAR-Ttherapy #Leukemia #CancerResearch #B_ALL #LeukemiaTreatment
Recently, a Chinese medical team published a notable study titled “Five-year outcome of CD19 combined with CD22 CAR-T cell therapy in B-ALL patients relapsed after allo-transplantation.” The research highlights the long-term efficacy of combined CD19 and CD22 CAR-T cell therapy in patients with relapsed acute B-lymphoblastic leukemia (B-ALL) after allogeneic hematopoietic stem cell transplantation (allo-HCT). This breakthrough has not only brought new hope to B-ALL patients but also attracted significant global attention.
**Background and Significance**
Acute B-lymphoblastic leukemia is a hematologic malignancy with poor prognosis, especially for patients who experience relapse after allo-HCT, where survival rates are significantly reduced. CAR-T cell therapy in China has shown increasingly positive results in treating B-ALL, particularly in targeting the CD19 antigen. However, the effects of targeting the CD22 antigen and its potential in combination with CD19 therapy are still under deeper investigation.
**Study Design and Methodology**
Based on a previous phase I clinical trial, this study involved a follow-up of 27 patients who had received CD19 CAR-T treatment. To comprehensively assess the treatment’s efficacy, the study also included three additional patients who experienced relapse with minimal residual disease (MRD) in the bone marrow. Although these patients did not meet the initial trial’s criteria, they received combined CD19 and CD22 CAR-T cell therapy under the same protocol. The CAR-T cells used in this study were second-generation designs created via lentiviral vector transfection.
**Study Results**
After a 5-year follow-up, the Chinese research team found that combined CD19 and CD22 therapy significantly improved patients’ long-term survival rates. Among the 30 patients who completed the combined therapy, the median follow-up time was 64.4 months. After two complete treatment cycles, patients maintained sustained remission. Survival analysis showed that the 3-year and 5-year overall survival rates reached 79% and 75%, respectively, with event-free survival rates of 54% and 50%. These results indicate that combined CD19 and CD22 CAR-T cell therapy offers substantial long-term efficacy for relapsed B-ALL patients.
**Conclusions and Future Outlook**
This study not only validates the potential of CAR-T cell therapy in hematologic malignancies but also provides new therapeutic insights for clinical practice. The combination of CD19 and CD22 holds promise for offering a more effective treatment option for B-ALL patients who relapse after allo-HCT, significantly improving their long-term survival rates.
As research in CAR-T cell therapy deepens, we may see more targeted approaches and optimized treatment protocols emerge. China’s active exploration and innovation in this field bring renewed hope to hematologic cancer patients worldwide, with the expectation that CAR-T therapy will further improve survival rates and quality of life for B-ALL patients in the near future.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#Immunotherapy #CD19CD22Combo #StemCellTransplant #ChinaMedicalResearch #Hematology #CancerBreakthrough #LongTermSurvival #BloodCancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Another New CAR-T Therapy in China is Fast-Tracking Approval, Targeting Pediatric Acute Lymphoblastic Leukemia(ALL) with a 100% Response Rate!
Another New CAR-T Therapy in China is Fast-Tracking Approval, Targeting Pediatric Acute Lymphoblastic Leukemia with a 100% Response Rate!

ALL
In recent years, CAR-T cell therapies from China have garnered global attention in the fight against cancer, and now another CAR-T product is about to hit the market. Priscabtagene Autoleucel Injection (pCAR-19B), developed by China’s Precision Biotech, is specifically designed for relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) and is currently under priority review for approval. It is poised to become China’s first CAR-T therapy specifically tailored for children and adolescents with acute leukemia.
This groundbreaking therapy has shown remarkable efficacy, achieving a 100% overall response rate in early clinical trials. For patients who no longer respond to conventional treatments, Priscabtagene Autoleucel offers a new lifeline. The therapy has demonstrated superior safety and efficacy in clinical settings, thanks to gene optimization and advanced vector systems that reduce side effects while enhancing effectiveness.
Currently, pCAR-19B has entered Phase II clinical trials, expanding its scope beyond children to include adult patients with relapsed leukemia. It is also being researched for the treatment of other malignant lymphomas, such as diffuse large B-cell lymphoma and follicular lymphoma. This signifies the vast potential of CAR-T therapies in China, promising more effective treatment options for blood cancer patients both domestically and globally.
Precision Biotech’s CAR-T therapy is expected to gain approval soon, contributing to global medical innovation while bringing new hope to countless patients.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
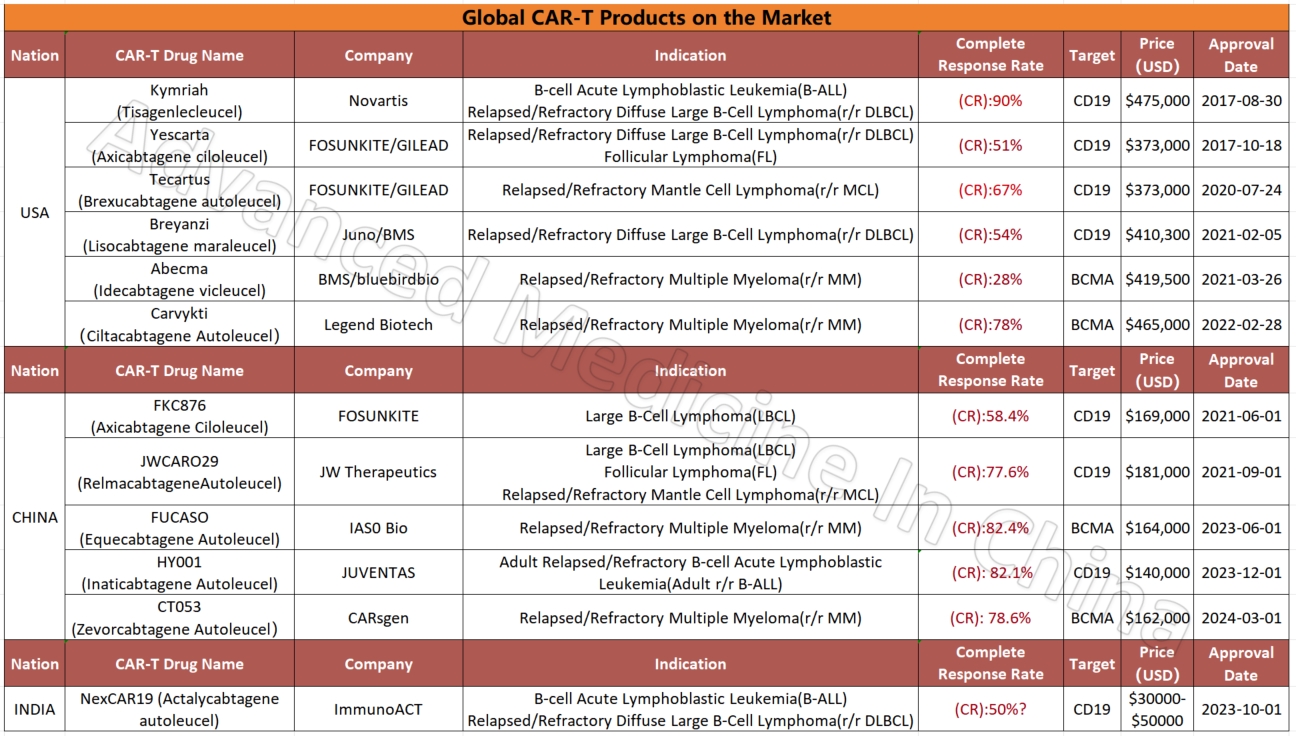
Global CAR-T Products on the Market and BCMA-Targeted CAR-T Products
Global CAR-T Products on the Market

CAR-T
Globally Approved BCMA-Targeted CAR-T Products
– Multiple Myeloma
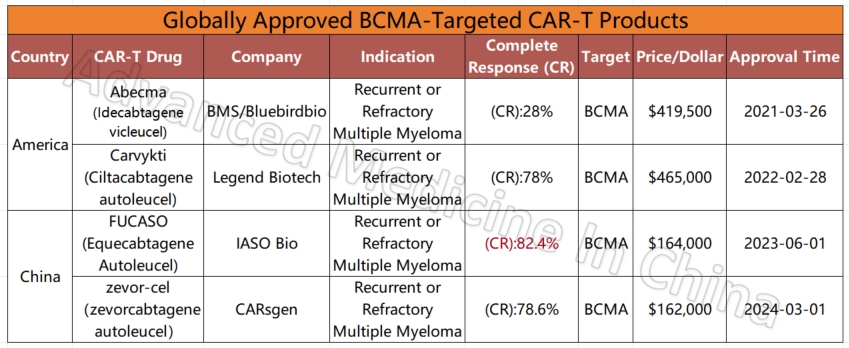
Multiple Myeloma

 To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Nature Medicine | New Breakthrough in China’s CAR-T Therapy: Significant Progress in Allogeneic CD5 CAR-T Treatment for Relapsed T-ALL
**Nature Medicine | New Breakthrough in China’s CAR-T Therapy: Significant Progress in Allogeneic CD5 CAR-T Treatment for Relapsed T-ALL**

T-ALL
#CAR-TTherapy #TALL #ALL #Leukemia #RelapsedLeukemia #TCellLeukemia #CD5 #CD7 #CART
Recently, a collaborative research study conducted by multiple Chinese medical and research institutions was published in *Nature Medicine*. This study, titled “Allogeneic CD5-specific CAR-T Therapy for Relapsed/Refractory T-ALL: A Phase 1 Trial,” demonstrated the potential of CD5 CAR-T cells in treating relapsed/refractory acute T-cell lymphoblastic leukemia (R/R T-ALL).
### Innovative Therapy Brings Hope
For a long time, patients with relapsed/refractory T-ALL have lacked effective treatment options and faced a poor prognosis. In recent years, CD7 CAR-T cell therapy has shown some efficacy in these patients, but CD7-negative relapse remains a significant challenge. This study focuses on evaluating the safety, efficacy, and pharmacokinetics of CD5 CAR-T cells, offering new insights for future treatments.
The study included 19 patients, most of whom had previously received CD7 CAR-T therapy. Results showed that this therapy was safe, with no dose-limiting toxicity observed, and adverse events were primarily manageable hematologic toxicity. In terms of efficacy, all patients achieved complete remission within 30 days of treatment. The study also demonstrated, for the first time, the persistence of CD5 CAR-T cells in the body and their ability to eliminate CD5+ T cells, indicating strong anti-tumor activity.
### Breakthrough in Treating Relapsed Patients
In addition to safety and efficacy, the research team explored the coexistence of CD7 CAR-T and CD5 CAR-T cells and studied immune cell changes in the patients. For those who relapsed after CD7 CAR-T treatment with CD7-negative cells, CD5 CAR-T offered a new salvage therapy, providing additional treatment options for such patients.
### Multidisciplinary Collaboration Drives Clinical Progress
Unlike CAR-T therapies for B-cell tumors, treating T-cell malignancies poses more challenges, particularly in controlling immune deficiencies. Dr. Jing Pan and her team conducted in-depth research on target selection and relapse mechanisms, while also focusing on balancing treatment safety and efficacy.
This study was made possible by the collaborative efforts of various medical institutions in China, collectively opening up a new therapeutic pathway for T-cell tumor patients.
### Looking Ahead
As innovations and advances in T-ALL treatment continue, the team plans to further research CD5 CAR-T therapy and collaborate with experts across various fields to optimize CAR-T treatment protocols, helping more patients with T-cell lymphoblastic tumors overcome their diseases.
This series of research achievements not only brings new hope to patients with R/R T-ALL but also provides valuable insights for the future development and optimization of CAR-T therapies. The team remains committed to a patient-centered approach, striving to drive continuous breakthroughs and innovations in T-cell tumor treatment.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#Immunotherapy #CancerTreatment #TALLResearch #LeukemiaBreakthrough #Hematology #CellTherapy #CancerInnovation #CancerResearch #ClinicalTrials #MedicalBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s New CAR-T Therapy ssCART-19: Exceptional Safety with Zero ICANS Incidence
China’s New CAR-T Therapy ssCART-19: Exceptional Safety with Zero ICANS Incidence

CARTTherapy
#ssCART19 #CAR_T #CART #ALL #ICANS #CNSL #CARTTherapy
CAR-T therapy has reached another breakthrough with the introduction of ssCART-19, a new treatment that leverages IL-6 gene silencing technology to significantly reduce side effects, particularly when treating relapsed/refractory acute lymphoblastic leukemia (r/r ALL). Unlike traditional CAR-T therapies, ssCART-19 drastically lowers the risks of severe cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
In the latest clinical trials, ssCART-19 demonstrated outstanding safety, with no patients experiencing ICANS, marking a milestone in CAR-T therapy safety. Among the 17 patients treated, 58.8% showed a significant increase in cell counts, and no cases of Grade 4 or higher CRS were reported. Additionally, ssCART-19 achieved an overall response rate (ORR) of 87.5% within three months, with nearly 63% of patients reaching complete remission.
The latest research also highlights that ssCART-19 outperforms traditional CAR-T products (cCART-19) in both safety and efficacy. Compared to conventional therapies, ssCART-19 significantly reduced the incidence of severe CRS, neutropenia, and ICANS, achieving an ORR of 91.5%. With these impressive results, ssCART-19 is expected to become a groundbreaking option for treating r/r ALL, offering new hope, especially for patients with central nervous system leukemia (CNSL).
Developed by a Chinese biopharmaceutical company, ssCART-19 has gained widespread recognition on the international stage, receiving Orphan Drug designation from the U.S. FDA and being listed as a breakthrough therapy in China. ssCART-19 offers a new treatment option for patients previously excluded from CAR-T therapy, greatly enhancing their chances of survival.
Stay tuned for more updates on how ssCART-19 is transforming cancer immunotherapy.

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CART #CancerTreatment #Leukemia #BreakthroughTherapy #InnovativeMedicine #Immunotherapy #CancerResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Haematologica Spotlight:A Chinese team published the largest-scale pediatric-style regimen treatment for adult Ph-negative ALL
🔬 *Haematologica Spotlight* 🔬
A Chinese team published the largest-scale pediatric-style regimen treatment for adult Ph-negative ALL
Acute Lymphoblastic Leukemia (ALL)
is a hematological malignancy characterized by the proliferation of immature lymphoid cells in the bone marrow, peripheral blood, and extramedullary sites. While the prognosis for childhood ALL is relatively favorable, with a 5-year overall survival rate of 80-90%, adult ALL poses greater challenges. Approximately 20-30% of adult acute leukemias are ALL, with about two-thirds being Philadelphia chromosome-negative (Ph-negative) ALL. Conventional adult ALL treatments have shown high remission rates but also high relapse rates and poor long-term survival.

Haematologica
Inspired by the success of pediatric ALL treatments, especially in adolescent and young adult patients, the Hematology Center at the
Chinese Academy of Medical Sciences and Peking Union Medical College Hospital
embarked on a groundbreaking journey. Their pioneering research, recently published in *Haematologica*, presents the results of the largest prospective cohort study in China investigating the efficacy of a pediatric-inspired regimen in adult Ph-negative ALL patients.
The study, led by Dr. Wang Jianxiang and his team,
evaluated the IH-2014 regimen’s effectiveness in 415 newly diagnosed adult Ph-negative ALL patients treated between April 2014 and December 2021, with a median age of 27 years (range 14-65 years) and a median follow-up of 40.8 months. The overall 5-year overall survival (OS) and disease-free survival (DFS) rates were 53.8% and 51.1%, respectively, with a chemotherapy-related mortality rate of 3.6%.
Patients achieving complete remission (CR)
after induction therapy with available bone marrow minimal residual disease (MRD) data were categorized based on diagnostic risk: standard-risk (SR) MRD-negative (<0.01%) in 73 patients (18.8%), SR MRD-positive (≥0.01%) in 69 patients (17.7%), high-risk (HR) MRD-negative in 120 patients (30.8%), and HR MRD-positive in 127 patients (32.6%). The 5-year OS rates for these groups were 82.6%, 58.7%, 58.3%, and 36.1%, respectively (P<0.001), with corresponding cumulative relapse rates of 24.2%, 41.1%, 36.3%, and 50.1% (P<0.001). Multivariate analysis identified age ≥40 years and MRD positivity after induction as independent adverse prognostic factors for OS and DFS.
In conclusion, the IH-2014 regimen demonstrates significant efficacy and good tolerability in adult Ph-negative ALL patients, with younger patients (<40 years) benefiting more prominently. Induction treatment response combined with MRD levels serves as a valuable prognostic indicator for long-term survival and relapse, guiding allogeneic hematopoietic stem cell transplantation decisions in patients achieving first complete remission (CR1).
This study marks a pivotal milestone in advancing adult ALL treatment strategies, showcasing China’s remarkable progress in the field of hematology. Stay tuned for more groundbreaking research from our team!
🎉To assess whether the condition is suitable for CAR-T therapy, you can submit Advanced Medicine in China for preliminary evaluation!
Email: doctor.huang@globecancer.com,
WhatsApp: +8613717959070
🌟 #Haematologica #LeukemiaTreatment #PediatricInspiration #ALL #AcuteLymphoblasticLeukemia #LymphoblasticLeukemia #Leukemia
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Breakthrough CAR-T Cell Therapy for R/R B-Cell ALL: A Game-Changer in Chinese Medical Innovation
Breakthrough CAR-T Cell Therapy for R/R B-Cell ALL: A Game-Changer in Chinese Medical Innovation

ALL- Acute Lymphoblastic Leukemia
The Fourth China Hematology Development Conference – CAR-T Frontier Forum and the First CAR-T Cell Immunotherapy Summit (JCIS), held on January 5, 2024, in Tianjin.
Potential Best-in-Class: Inaticabtagene Autoleucel Redefining Long-term Outcomes for R/R B-ALL
Professor Ma Jun from the Harbin Institute of Hematology and Oncology shared notable progress in immunotherapy and cell treatment for Chinese ALL. Previously, the overall complete response (CR) rate for adult R/R ALL treatment in China was approximately 40%, with a mere 11% 3-year survival rate. The introduction of CAR-T cell therapy has been a paradigm shift, altering the long-term outcomes for R/R B-ALL patients.

Inaticabtagene Autoleucel demonstrates superior efficacy:
Higher overall response rates (ORR) at 3 months and beyond, with median duration of response (DOR) and overall survival (OS) surpassing other products.
Patients treated with Inaticabtagene Autoleucel exhibit similar long-term benefits in OS, whether or not they undergo subsequent transplantation.
Inaticabtagene Autoleucel boasts enhanced safety:
Lower incidence rates of grade 3 cytokine release syndrome (CRS), grade 3 immune effector cell-associated neurotoxicity syndrome (ICANS), and infusion-related mortality compared to other CAR-T products.
Moreover, in minimal residual disease-positive (MRD+) B-ALL patients, CAR-T cell therapy has shown significant progress. It eradicates MRD, improves survival rates, and may serve as a first-line consolidation therapy for CR patients, aiding in:
Higher remission rates
Reduced transplant requirements, mitigating transplant-related complications
Maintenance of long-term remission for those unsuitable for allo-HSCT or unwilling to undergo it
Improved overall survival
Lower intensity and duration of intensive chemotherapy, leading to shorter treatment times and enhanced compliance.
The Future Outlook: Believing in the potential of CAR-T cell therapy, it is anticipated that this innovative treatment will extend hope to currently incurable diseases such as solid tumors and brain tumors. The strides made in Chinese medical innovation, exemplified by Inaticabtagene Autoleucel, signal a promising future for the global landscape of CAR-T cell therapy.
#CARTRevolution #InaticabtageneAutoleucel #HematologicCancerTherapy #ClinicalBreakthrough #ChinaHematologyConference #JCIS #BAllTreatment #MedicalAdvancements #CancerResearch #TreatmentInnovation #InaticabtageneAutoleucel #Autoleucel #ChineseCART #CARTTherapy #cancer #Bloodcancer #MedicineinCHINA #Medicaltourismo #Advancedmedicine #cancertherapy #leukemia
