What is Chimeric Antigen Receptor (CAR) T-cell Therapy?
T-cells or T-lymphocytes are a type of white blood cells in our immune system. T-cells have the capacity to recognise abnormal cells or any cells infected by viruses in the body, and then destroy these abnormal cells.
However, T-cells may sometimes fail to recognise or eliminate these threats in the body, such as in the case of cancer.
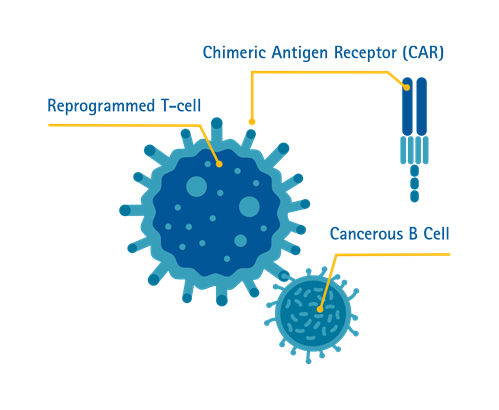
Chimeric Antigen Receptor (CAR) T-cell Therapy is a form of immunotherapy where T-cells are taken from the patient’s blood and modified in a laboratory setting to enable the T-cells to identify and destroy specific cancer cells. The modified T-cells are then reinfused into the patient. Once back inside the patient’s body, the modified T-cells will be able to detect the cancer cells and destroy the cancer by harnessing the body’s own immune response.
Singapore is the first country in Southeast Asia to offer the treatment^.
^Source: CNA
What conditions can it treat?
CAR T-cell Therapy is particularly effective for patients diagnosed with relapsed aggressive forms of Acute Lymphoblastic Leukemia (ALL) and relapse of Non-Hodgkin Lymphoma such as Diffuse Large B-cell Lymphoma (DLBCL), especially when at least two prior treatment regimens have failed to produce the desired outcomes.
Who is eligible?
Selected groups of patients are eligible for CAR T-cell Therapy. They include:
- Children and young adult patients from 2 to 25 years old with B-cell Acute Lymphoblastic Leukemia (ALL) that is resistant, and where a relapse has occurred subsequently or post-transplant.
- Adults with Diffuse Large B-cell Lymphoma (DLBCL) who have not benefited from at least two types of standard treatment.
The following groups of patients may not be eligible for CAR T-cell Therapy:
- Patients with intracranial hypertension or unconsciousness
- Patients with respiratory failure
- Patients with disseminated intravascular coagulation
- Patients with hematosepsis or uncontrolled active infection
Receiving CAR T-cell Therapy
How does it work?
The patient will first undergo screening and a series of tests to determine if CAR T-cell Therapy is an appropriate treatment option for their disease, and to ensure that the patient is fit to undergo treatment.
Step 1. Collecting the T-cells
White blood cells, which include T-cells, will be extracted from the patient’s blood using a procedure called leukapheresis. During this procedure, two intravenous infusion (IV) lines will be inserted into the patient: blood is extracted through one line, to allow the white blood cells to be separated out and extracted, while the rest of the blood is returned to the patient’s body through the second line.
Patients will be asked to lie down or be seated on a reclining chair while the procedure is being carried out.
Step 2. Making the CAR T-cells
When the white blood cells have been extracted, the T-cells will be separated out and sent to the laboratory to be altered. This alteration is carried out by adding the specific Chimeric Antigen Receptor (CAR) gene to the T-cells, hence modifying them into CAR T-cells. These cells will then be grown and multiplied in the laboratory.
Under normal circumstances, it can take 2–3 weeks to produce the adequate number of CAR T-cells required for CAR T-cell Therapy.
Step 3. Receiving the CAR T-cell Infusion
When enough CAR T-cells have been produced, the product will be shipped back to the hospital to be infused into the patient.
A few days prior to the CAR T-cell infusion, the patient may be given chemotherapy to lower the number of other immune cells in the body and prepare the body to receive the CAR T-cells. This provides the newly infused CAR T-cells a better chance to be ‘activated’ to fight the cancer. Generally, the chemotherapy is less intense to ensure there are cancer cells remaining for the CAR T-cells to ‘activate’ effectively.
Once the CAR T-cells begin binding with cancer cells in the body, they will begin to increase in number and destroy even more cancer cells.
Step 4. Recovery
Patients receiving CAR T-cell Therapy will have an early recovery period of approximately 6–8 weeks. During this period, patients will be monitored for any side effects and assessed on treatment response.
What does the recovery process look like?
Recovery typically takes 2–3 months from the CAR T-cell infusion. Patients will be admitted during the first 2–3 weeks to recover from any side effects of chemotherapy before they can be discharged.
Following discharge, patients will have to go for regular outpatient appointments to monitor side effects and clinical responses to treatment.
Side Effects of CAR T-cell Therapy
What are the side effects?
One common side effect of CAR T-cell Therapy is Cytokine Release Syndrome (CRS), which is a multisystemic disease resulting from the effects of CAR T-cells at work and elimination of cancer cells.
Side effects of CRS include:
- High fever and chills
- Difficulty breathing
- Nausea, vomiting and/or diarrhoea
- Feeling dizzy and lightheaded
- Headaches
- Fast heartbeat
- Fatigue
- Muscle and/or joint pain
CRS can develop many weeks after infusion, but most commonly develop within two weeks after infusion. The severity of CRS is not correlated with the response to CAR T-cell Therapy.
Another common side effect is immune effector cell-associated neurotoxicity syndrome (ICANS), which affects the central nervous system of the patient.
CRS and ICANS are well recognised side effects that are highly treatable and can be managed by a trained clinical care team.
Benefits & Challenges
What are the benefits and challenges?
CAR T-cell Therapy offers patients with blood cancers a potential life saving treatment option in the event that their disease is not controlled by standard chemotherapy, targeted therapy or bone marrow transplantation.
However, as it is a relatively new area of cell therapy, there are some challenges to consider such as the selection of patients who will benefit from this treatment, their level of medical fitness, the timing of cell collection, logistical concerns, and the risk–benefit ratio of treatment for the individual patient.
- For-profit or non-profit?
- Size
- Cost
- Average length of stay
- Ownership
- Accredited by
- Certifications
- Established_In
- Hospital_Grade