 1 year ago
1 year ago
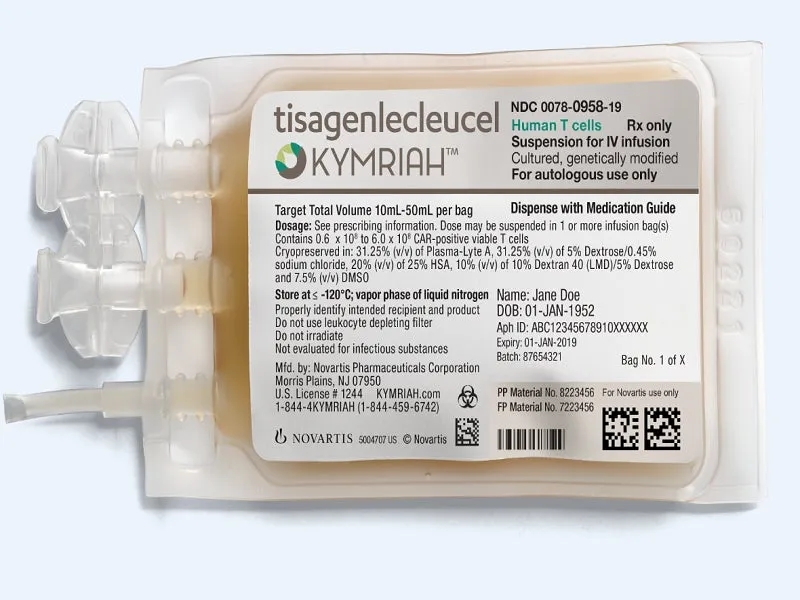
Kymriah Drug
Kymriah Drug Kymriah (Tisagenlecleucel) is a type of cancer treatment known as CAR T-cell therapy. It involves modifying a patient’s own T cells to target and kill cancer cells. Here’s ...
 1 year ago
1 year ago
Kymriah Drug Kymriah (Tisagenlecleucel) is a type of cancer treatment known as CAR T-cell therapy. It involves modifying a patient’s own T cells to target and kill cancer cells. Here’s ...
 1 year ago
1 year ago
**2024EHA | Professor Huang He: Targeting CD7 Universal CAR-T Therapy Shows Significant Efficacy and Good Safety in Treating R/R CD7+ T-ALL/LBL Patients** The 29th Annual Meeting of the European H...
 1 year ago
1 year ago
May 1, 2018 Kymriah DLBCL Approval in the U.S. FDA On May 1, 2018, the FDA approved Kymriah for the treatment of adult patients with relapsed or refractory large B-cell lymphoma (LBCL). Kymriah is ...
 1 year ago
1 year ago
Kymriah Cell Therapy of CAR-T Kymriah, also known by its generic name tisagenlecleucel, is a groundbreaking cell therapy used to treat certain types of blood cancers. Developed by Novartis, Kymriah...
 1 year ago
1 year ago
Kymriah CD19-Targeted CAR-T Therapy Kymriah (tisagenlecleucel) is a groundbreaking CAR-T (Chimeric Antigen Receptor T-cell) therapy developed for the treatment of certain types of blood cancers. He...
 1 year ago
1 year ago
### China’s Breakthrough in CAR-T Therapy for CNS Lymphoma Lymphoma In a pioneering effort to improve outcomes for patients with refractory/relapsed central nervous system lymphoma (CNSL), a multi-...
 1 year ago
1 year ago
China’s Urological Technology Leads the Way! Over 100 International Doctors from More than 40 Countries Come to Learn China’s Urological ## Innovative Breakthroughs in Urology ChinaR...
 1 year ago
1 year ago
Kymriah CAR-T Cell Therapy: A Comprehensive Overview Introduction Kymriah (tisagenlecleucel) represents a groundbreaking advancement in the field of immunotherapy. It is a chimeric antigen receptor...
 1 year ago
1 year ago
Kymriah Approved Indications Kymriah (tisagenlecleucel) represents a groundbreaking advancement in the field of cancer immunotherapy. As a chimeric antigen receptor T-cell (CAR-T) therapy, Kymriah ...
 1 year ago
1 year ago
Kymriah Approval FDA On April 22, 2020, Novartis announced that the U.S. Food and Drug Administration (FDA) granted Kymriah® (tisagenlecleucel) Regenerative Medicine Advanced Therapy (RMAT) designa...
 1 year ago
1 year ago
Kymriah Approval DLBCL As of May 1, 2018, the FDA has approved Novartis’ CD19-targeted CAR-T therapy tisagenlecleucel (Kymriah) for the treatment of adult patients with relapsed or refractory...
 1 year ago
1 year ago
Kymriah Approval Date Introduction to Kymriah Kymriah (tisagenlecleucel) is a groundbreaking chimeric antigen receptor T-cell (CAR-T) therapy developed by Novartis. It represents a novel approach t...
By using our site, you agree to our Terms and Conditions and Privacy Policy.Advanced Medicine In China does not provide medical advice, diagnosis, or treatment. The information provided on this site is designed to support, not replace, the relationship that exists between a patient/site visitor and his/her existing physician.
© Copyright 2023 Advanced Medicine In China. All rights reserved.