Breakthrough in Survival for Late-Stage Cancer Patients with Chinese CAR-T Therapy
Whether it’s multiple treatment failures or recurrent tumors with systemic metastasis, miraculous remissions are still achieved after undergoing treatment! Chinese CAR-T therapy has brought a brand-new hope for various late-stage cancers such as gastric, colorectal, pancreatic, and liver cancers.
The renowned CAR-T therapy is almost universally recognized, and its successful development has instilled hope for the ‘cure’ of cancer. Presently, nine CAR-T therapies available in both China and the United States exhibit an overall remission rate exceeding 80%, with these treatments confirmed to significantly trigger responses in hematologic malignancies. Meanwhile, scientists worldwide are fervently working to develop CAR-T therapies capable of exerting robust anti-cancer effects in solid tumors as well.
Finally, Chinese researchers have achieved a significant breakthrough. In 2023, multiple research achievements of the National Research CAR-T have made headlines in leading international oncology conferences and authoritative journals, causing a tremendous sensation!
- Pancreatic Cancer: Tumors significantly shrink or completely disappear! National Research CAR-T Challenges the ‘Cancer King’
Tumors of two pancreatic cancer patients who had failed chemotherapy and experienced systemic metastasis have notably shrunk, with some metastatic lesions disappearing entirely! China has successfully developed CAR-T therapy targeting solid tumors, effectively combating pancreatic cancer. Numerous patients have successfully applied through the Global Oncologists Network to become the first batch of ‘trailblazers’ receiving CAR-T therapy. The good news is that CAR-T therapy is currently recruiting various cancer patients in China, including those with pancreatic cancer!
In November 2023, the prestigious international medical journal ‘Hematology and Oncology Journal’ published new cases of solid tumor CAR-T therapy treating two patients with metastatic pancreatic cancer. The reason for the excitement is that both patients had failed chemotherapy, had systemic metastasis, and were late-stage refractory patients with no better treatment options. After receiving CAR-T therapy, one patient’s tumor significantly shrank, while all lung metastatic lesions in the other patient unexpectedly disappeared, achieving complete remission (CR), which is highly encouraging!

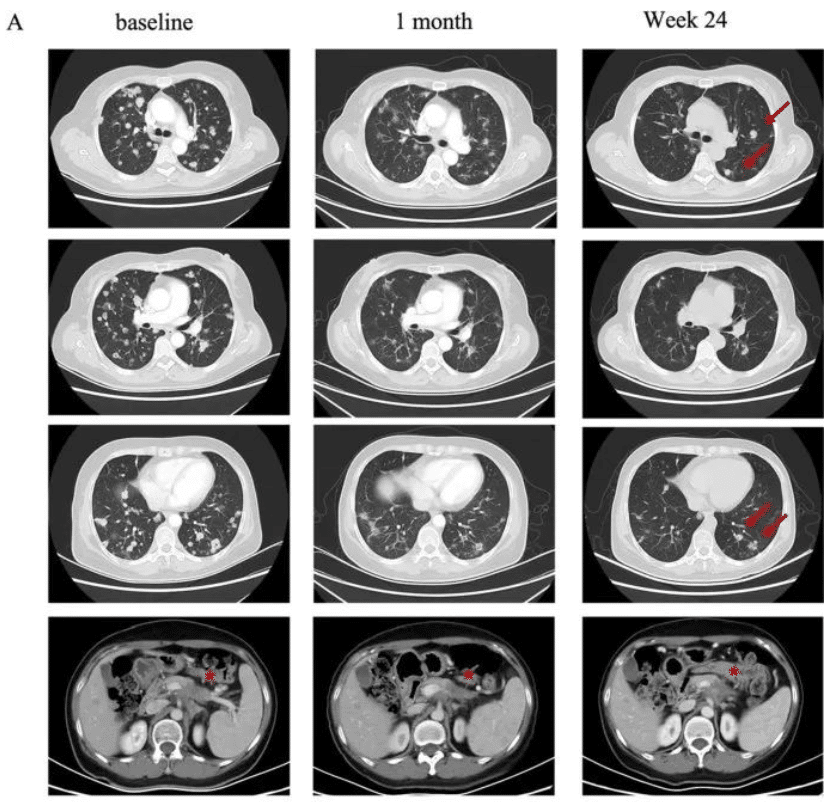
A 58-year-old female pancreatic cancer patient with ineffective multiple treatment regimens and continuous tumor progression. In 2021, she joined a clinical trial for CAR-T therapy. In September 2021, she received a dose of 250×10^6 CT041 cells. The target lesions shrunk significantly by more than 30%, achieving a partial response (PR), and there was also a significant reduction in lung metastasis!
A 75-year-old female pancreatic cancer patient developed lung metastasis just five months after surgery, with chemotherapy proving ineffective and the condition rapidly progressing. After receiving a dose of 250 × 10^6 CT041 cells, a follow-up just four weeks later showed a significant reduction in tumors, and subsequent lung metastatic lesions disappeared, achieving complete remission (CR)! Until the last follow-up in July 2023, the tumor remained well-controlled.
Dr. Raffaele Baffa, Chief Medical Officer of SciClone Pharmaceuticals, stated: ‘We believe that CT041 has the potential to change the treatment landscape for pancreatic cancer and other solid tumors, bringing new hope to patients and their families.
- Gastric Cancer: Objective response rate reaches up to 60%! Complete remission observed in late-stage gastric cancer
In the first-ever disclosed clinical study results of Claudin18.2 CAR-T therapy in the United States, a fortunate patient experienced the complete disappearance of target lesions, achieving full remission!
Claudin18.2 is a pan-tumor target expressed in various epithelial tumors, particularly high in gastric and pancreatic cancers. In gastric or gastroesophageal junction cancer, up to 60% of patients exhibit high expression of Claudin18.2. Based on this, Chinese researchers developed the world’s first CAR-T cell therapy targeting Claudin18.2—CT041. At the 2022 ASCO conference, research teams from China and the United States presented remarkable research outcomes of this therapy for gastric cancer globally, signifying that the Chinese team pioneered breaking the bottleneck of CAR-T therapy in solid tumors! China’s CAR-T therapy is shining worldwide! The preliminary clinical data from the United States was disclosed for the first time. As of May 6, 2022, 14 CLDN18.2 positive patients have been enrolled (including 5 gastric/gastroesophageal junction cancer and 9 pancreatic cancer patients). Notably, these patients had previously received third-line treatment, with no better clinical options available. In the gastric/gastroesophageal junction cancer subgroup, an impressive objective response rate (ORR) of up to 60% was reported, with one patient achieving complete remission (CR). Additionally, 80% (4 out of 5) of the patients remained stable, with varying degrees of tumor reduction.
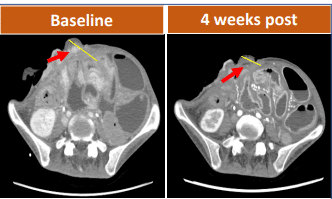
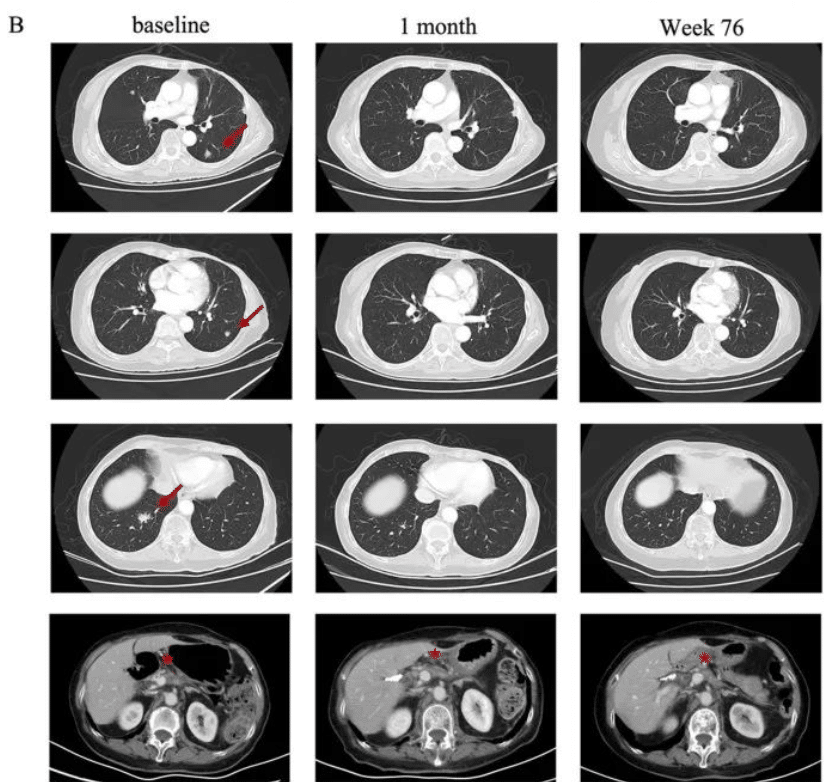
A 48-year-old female with advanced gastric adenocarcinoma, with a 7.6cm tumor spreading extraperitoneally, showed significant tumor reduction after 4 weeks of CAR-T treatment.
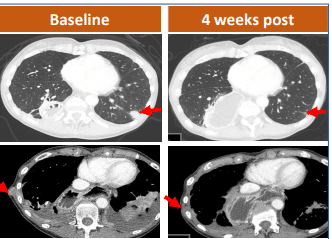
A 62-year-old female with gastric adenocarcinoma and lung metastasis showed complete disappearance of one target lesion in the lungs after just 4 weeks of CAR-T treatment, while two others markedly decreased in size. CT041 is the only CLDN18.2 CAR-T cell therapy approved for clinical trials in both China and the United States.
In 2020, CT041 was granted orphan drug designation by the FDA for the treatment of gastric/gastroesophageal junction (GC/GEJ) cancers, and in 2021, it received the orphan drug designation from the EMA for gastric cancer treatment.
The good news is that numerous patients with gastric and pancreatic cancers and other gastrointestinal tumors have joined this clinical trial conducted in China through the Global Oncologists Network, gaining more treatment opportunities. Currently, related experiments are still recruiting.
Even better news is that the results of phase 1b clinical trial ELIMYN18.2, using chimeric antigen receptor T-cell therapy targeting CLDN18.2 for advanced gastric and pancreatic cancer patients, are set to be announced at the 2024 ASCOGI. Let us eagerly await the latest surprises regarding this miraculous therapy for gastric and pancreatic cancers.
- Liver Cancer: Complete remission lasting up to 8 years! GPC3 CAR-T brings ‘cure’ miracle for liver cancer patients!
Two patients with multiple treatment failures, relapse, and rapid progression of advanced liver cancer achieved complete remission after receiving a novel immunotherapy! What’s even more inspiring is that these two patients recently revisited a renowned oncology center in Shanghai for a check-up, confirming a sustained complete remission lasting 7 and 8 years! This signifies that the CAR-T therapy developed successfully in China for solid tumors enabled clinical ‘cure’ in two late-stage liver cancer patients!
GPC3 is a cancer embryonic antigen involved in cell proliferation, differentiation, migration, and apoptosis. It is scarcely expressed in normal tissues but present in 70-80% of hepatocellular carcinomas (HCC). Due to its tumor specificity, GPC3 is considered a ‘golden’ target capable of precisely targeting liver cancer cells!
Based on this, Chinese researchers have developed CAR-T cell therapy targeting GPC3 and TCR-T cell therapy, achieving significant breakthroughs. Recently, a heartening piece of news made headlines across major oncology media platforms and cancer communities. The publication of two cases of long-term disease-free survival in patients with advanced hepatocellular carcinoma (HCC) treated with innovative CAR-GPC3 T cell therapy from the National Research Center for Translational Medicine (NRCTM) was featured in the renowned international immunology journal ‘Cancer Communications,’ causing a significant stir!
These fortunate patients had already presented with inferior vena cava tumor thrombus upon enrollment in 2015, with one patient additionally experiencing retroperitoneal lymph node metastasis. These advanced-stage patients had no better treatment options available clinically, with a very poor prognosis. To everyone’s delight, both patients maintained a tumor-free status during long-term follow-ups after receiving local treatment and CAR-T cell combined therapy. Recently, the patients returned to the hospital for re-examination, confirming a complete remission of tumors lasting 7 and 8 years.
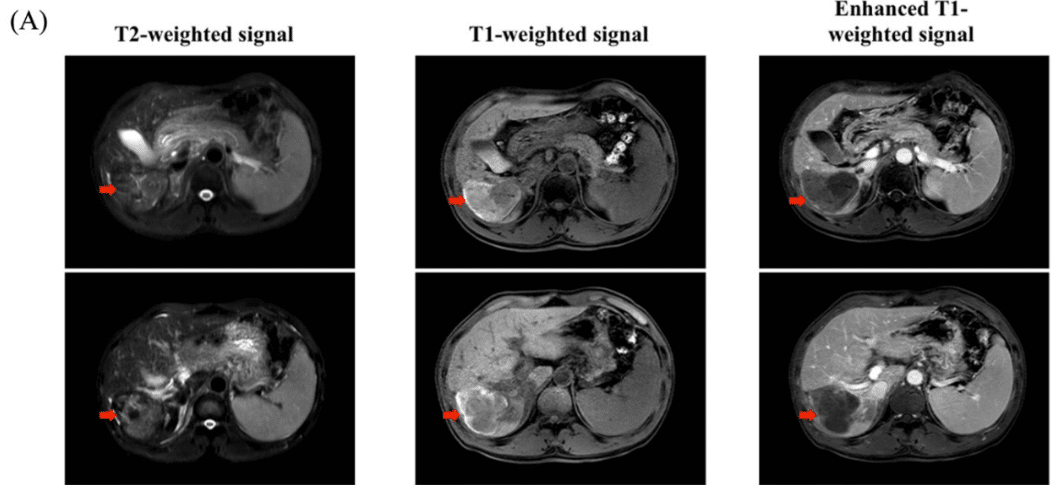
A 50-year-old male with hepatocellular carcinoma experienced disease progression after multiple treatments and presented with an inferior vena cava tumor thrombus, critical condition. After receiving CAR-GPC3 T cell therapy for 6 months, the re-examination results showed normal AFP levels, and imaging scans revealed no signs of tumors. He has been cancer-free for 8 years (from July 2015 to August 2023) and has returned to a normal life.
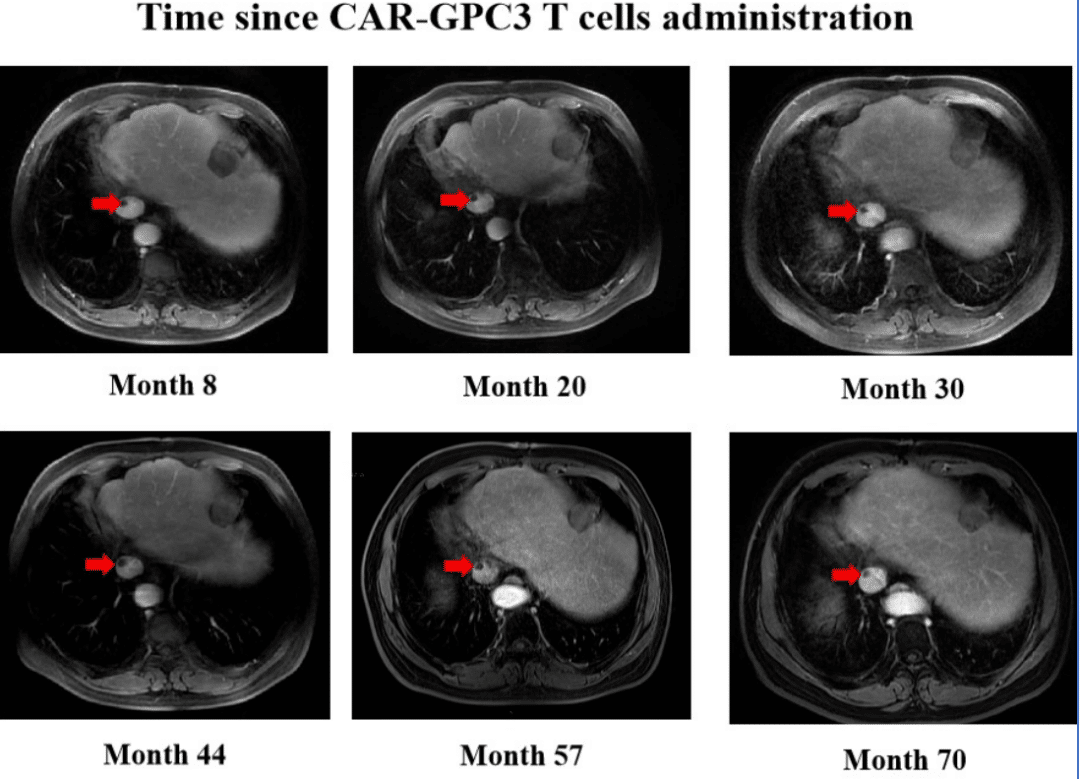
A 54-year-old male with hepatocellular carcinoma relapsed 3 months after surgery, with inferior vena cava tumor thrombus and retroperitoneal lymph node metastasis following multiple treatments. Clinically, there were no better treatment options available, and he participated in a CAR-GPC3 T cell clinical trial in China. Within just 2 weeks, he achieved complete remission and has now achieved over 7 years of tumor-free survival (from July 2016 to August 2023). Furthermore, during the 7-8 years, both patients only received oral antiviral drugs for hepatitis B and did not receive any other cancer treatment.
- Colorectal Cancer: Significant tumor shrinkage in half of the patients, GCC19CART lights up hope for colorectal cancer patients
GCC/GUCY2C plays a crucial role in the fluid and ion homeostasis of the gastrointestinal tract. About 80% of colorectal cancer patients exhibit positive GCC expression in their tumors, making it a ‘golden’ target capable of precisely targeting colorectal cancer cells!
Based on this, Chinese researchers have developed CAR-T cell therapy targeting GCC called GCC19CART.
Recently, the GCC19CART therapy released its latest research data. This was a multicenter dose-escalation clinical trial initiated by researchers in China. Twenty-one subjects were randomly assigned to two dosage groups. All these patients had received second-line or higher treatment before enrollment. Thirteen subjects were included in the low-dose group (1×10^6 CART/kg), and eight subjects were included in the medium-dose group (2×10^6 CART/kg). The objective response rate in the low-dose group was 15.4% (2/13), and in the medium-dose group, it was 50% (4/8). The medium-dose group’s median overall survival (mOS) has exceeded 24 months, significantly better than the FDA-approved standard third-line drugs (mOS is only 6-8 months). The preliminary results of this study show that GCC19CART demonstrates dose-related good initial clinical efficacy and manageable safety in patients with recurrent/refractory metastatic colorectal cancer.
Good news! Multiple domestic cancer centers have initiated CAR-T cell therapy clinical trials.
Although the CAR-T therapy in the aforementioned study is in the stage of solid tumor clinical trials, with the advancement in CAR-T generations, significant improvements have been made in proliferation, cytokine release, etc. Numerous clinical trials have applied CAR-T in solid tumor treatment, and fortunate patients have achieved complete remission, bringing a warm spring for late-stage solid tumor patients!
These studies hope to bring new hope to fellow patients. The good news is that these cutting-edge international T-cell therapies are recruiting various solid tumor patients for clinical trials. Those who seek help with CAR-T, TCR-T, TIL, and other cell therapies, both domestically and internationally, and are financially capable can contact us.
If cancer patients mentioned above want to test related targets or understand details, they can contact us for evaluation.
Before the emergence of cell therapy, solid tumors, including late-stage gastric adenocarcinoma and pancreatic cancer, were typically treated with surgery, radiation, and chemotherapy. Gastric adenocarcinoma accounts for 95% of malignant gastric tumors, while pancreatic cancer is the most malignant tumor among common cancers, with a median survival period and a 5-year survival rate much lower than other tumors, earning it the title of ‘king of cancers.’ However, most patients experience local recurrence or metastasis after surgery. Additionally, these malignant tumors are not highly sensitive to radiation or chemotherapy. Therefore, based on current standard therapies, treatment outcomes are not ideal, and the prognosis is extremely poor. The emergence of immunotherapy will bring long-term survival hopes and miracles to more advanced-stage patients.
We firmly believe that with the establishment of a comprehensive cell immunotherapy regulatory system, the country will open the door to cell immunotherapy, benefiting more cancer patients. Our country’s cell immunotherapy will step onto the international stage with great strides!
Breakthrough in Survival for Late-Stage Cancer Patients with Chinese CAR-T Therapy
whatsapp:+8613717959070
