New Generation CAR-T Cell Therapy AT101: Phase I Clinical Trial Shows 100% Complete Remission Rate!
New Generation CAR-T Cell Therapy AT101: Phase I Clinical Trial Shows 100% Complete Remission Rate!
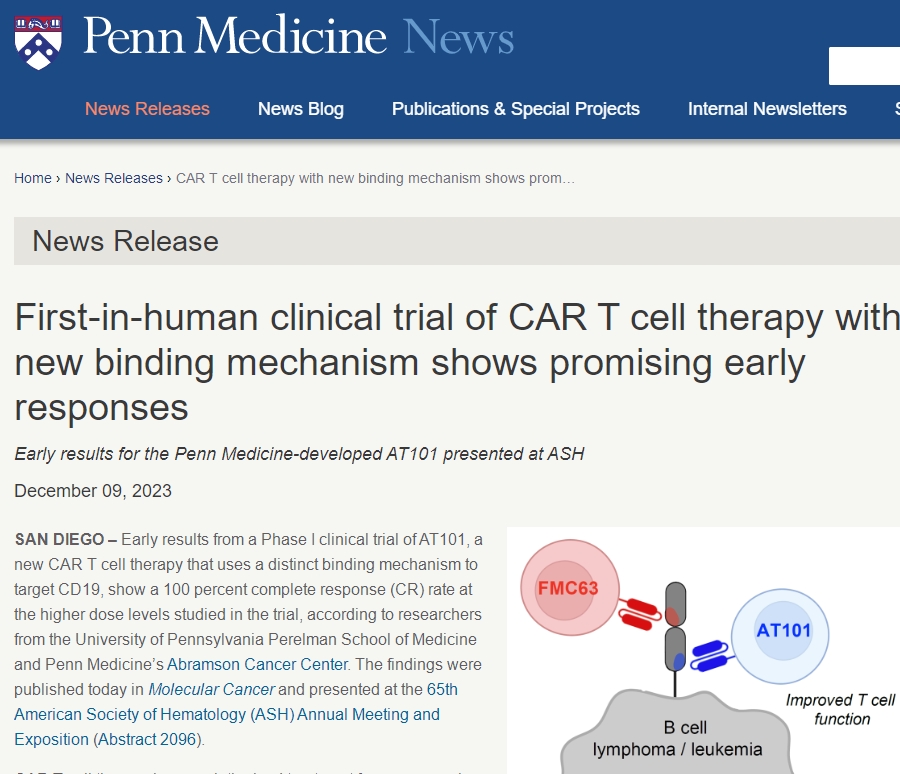
Researchers recently released exciting news: the Phase I clinical trial of their newly developed CAR-T cell therapy, AT101, demonstrated a 100% complete remission rate among patients receiving high-dose treatment. This groundbreaking achievement was published in the latest “Molecular Cancer” journal and gained significant attention at the 65th American Society of Hematology (ASH) Annual Meeting.
Despite recent FDA investigations into the safety of CAR-T cell therapy, it remains the most promising choice for blood cancer patients who have tried other treatment methods unsuccessfully. CAR-T cell therapy has fundamentally changed the treatment landscape for many blood cancer patients. While some patients show long-term responses to it, others do not.
The new CAR-T cell therapy, AT101, developed by researchers from the Perelman School of Medicine and the Abramson Cancer Center at the University of Pennsylvania, exhibited highly encouraging results due to its design targeting a new epitope of CD19 through a unique binding mechanism. Most currently approved CD19 CAR-T cell therapies target the same epitope, yet many patients eventually relapse. AT101, by targeting a different CD19 epitope, shows faster action rates and aims to reduce the failure rate of CAR-T cell therapy while improving clinical efficacy.
In this Phase I clinical trial, 14 patients with relapsed or refractory B-cell non-Hodgkin lymphoma (NHL) received treatment. Nine out of twelve patients achieved a complete remission status, indicating a total remission rate of 91.7%, with eight patients achieving complete remission. These patients did not experience cancer relapse, and the drug showed promising safety results.
While further research and larger-scale clinical trials are necessary, these early results instill considerable confidence. The success of AT101 holds promise for bringing new hope to blood cancer patients, especially those who previously did not receive effective CAR-T cell therapy. This study also aims to expand to a broader patient population, offering more treatment options and possibilities.
#CARTTherapy #BloodCancerTreatment #AT101Research #CancerRemission #MedicalBreakthrough #ASHAnnualMeeting #ClinicalTrialSuccess #UniversityResearch #CancerResearchUpdate #HopeForPatients #AT101 #BloodCancer #CancerTreatment
