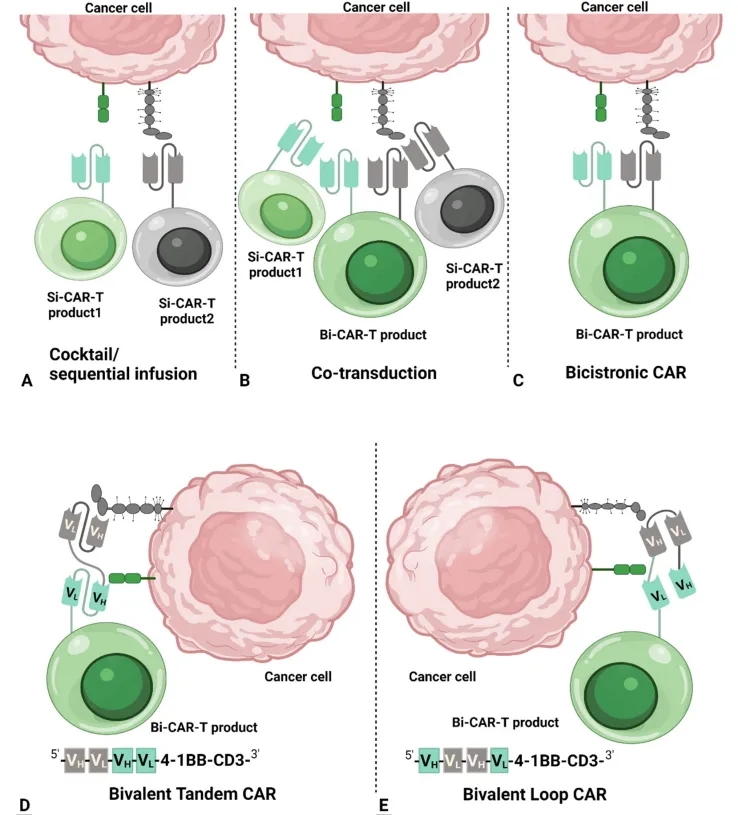
Hematological Neoplasms
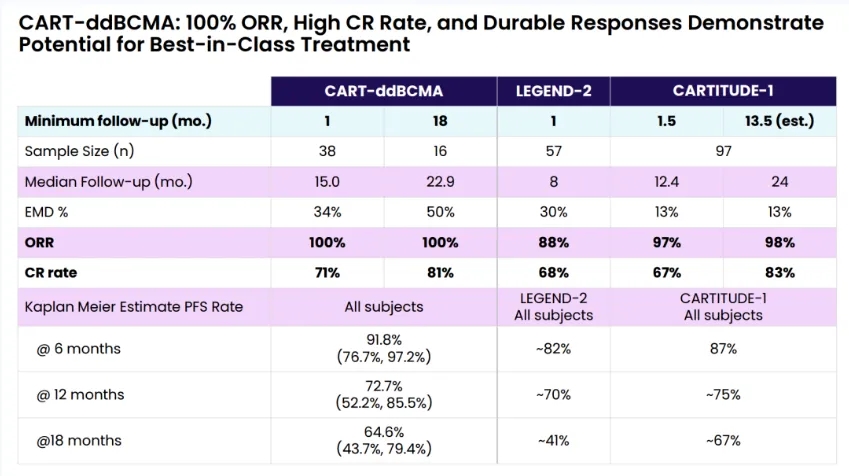
On December 8, 2023, Arcellx released new clinical data from the phase 1 expansion study of CAR-T-ddBCMA [now called anitocabtagene autoleucel (anito-cel)].
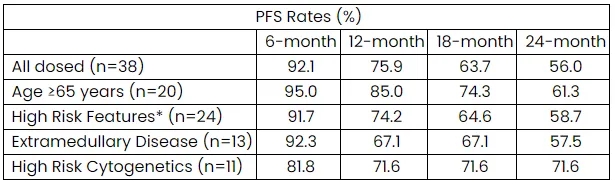
At the end of 2023, Arcellx, an immune cell therapy company, revealed the latest clinical data from the Phase 1 extension study of its cell therapy product.

On April 5th, 2024 local time, Johnson & Johnson announced that the U.S. FDA has expanded the label for Johnson & Johnson and Legend Biotech’s multiple myeloma CAR-T therapy, making it an earlier treatment option for patients.

🌈Treatment Across Continents: American Myeloma Patient Finds New Life in China!🌈 🌟Mr. C from California, USA It’s yet another heartening piece of news as Mr. C from California, USA, undergoes cross-border treatment at Jiangsu Province People’s Hospital in China, successfully overcoming multiple myeloma and embracing a new lease on life! After meticulous treatment spanning Read More

On February 23, 2024, Legend Biotech announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended the approval of expanding the indication for CARVYKTI® (cilta-cel, Ciltacabtagene Autoleucel) to include adult patients with relapsed and refractory multiple myeloma who have received at least one prior line of therapy (including an immunomodulatory agent and a proteasome inhibitor), and have progressed on the last line of therapy and are refractory to lenalidomide.

Johnson & Johnson and Legend Biotech’s jointly developed chimeric antigen receptor (CAR) T-cell therapy Carvykti (ciltacabtagene autoleucel, cilta-cel) has been approved by the U.S. FDA for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who have previously received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD), and are refractory to lenalidomide.

On April 5, 2024, local time, Legend Biotech (NASDAQ: LEGN) announced in Somerset, New Jersey, USA, that the U.S. Food and Drug Administration (FDA) has approved CARVYKTI® (Ciltacabtagene Autoleucel, cilta-cel) for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who have received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD), and are refractory to lenalidomide1.

On February 23, 2024, Legend Biotech announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended the approval of an expanded indication for Carvykti (cilta-cel, Ciltacabtagene Autoleucel) to include adult patients with relapsed and refractory multiple myeloma who have received at least one prior line of therapy (including an immunomodulatory agent and a proteasome inhibitor) and have progressed on their last line of therapy and are refractory to lenalidomide.

Carvykti (Ciltacabtagene Autoleucel, cilta-cel) is a chimeric antigen receptor T-cell (CAR-T) immunotherapy targeting the B-cell maturation antigen (BCMA).