Yescarta CAR-T Cell Therapy is Safe and Effective for Relapsed/Refractory Indolent Non-Hodgkin Lymphoma (iNHL) Patients: 3-Year Follow-Up Analysis from the ZUMA-5 Study
Yescarta CAR-T Cell Therapy is Safe and Effective for Relapsed/Refractory Indolent Non-Hodgkin Lymphoma (iNHL) Patients: 3-Year Follow-Up Analysis from the ZUMA-5 Study
Yescarta (Axicabtagene ciloleucel, axi-cel) is an autologous anti-CD19 chimeric antigen receptor (CAR)-T cell therapy approved for relapsed/refractory (R/R) follicular lymphoma (FL). This approval was also supported by the phase 2, multicenter, single-arm ZUMA-5 study of Yescarta in patients (n=104) with R/R indolent non-Hodgkin lymphoma (iNHL), including FL and marginal zone lymphoma (MZL). In the preliminary analysis (median follow-up 17.5 months), the overall response rate (ORR) was 92% (complete response [CR] rate: 74%).
This article reports the long-term follow-up results of the ZUMA-5 study. Eligible R/R iNHL patients underwent leukapheresis after receiving ≥2 prior lines of therapy, followed by lymphodepleting chemotherapy and infusion of Yescarta (2 × 106 CAR-T cells/kg). The primary endpoint was ORR, which the researchers evaluated in all enrolled patients (intention-to-treat) in this analysis.
In FL (n=127) and MZL (n=31) patients with a median follow-up of 41.7 months and 31.8 months, respectively, the ORRs were comparable to the initial analysis (FL: 94%; MZL: 77%). The median progression-free survival (PFS) in FL was 40.2 months, and the median was not reached in MZL. The median overall survival (OS) was not reached for either group. Since the previous analysis, ≥grade 3 adverse events (AEs) of special interest occurred primarily in patients with recent exposure. Clinical and pharmacodynamic results correlated negatively with recent bendamustine exposure and high baseline metabolic tumor volume.
Background
R/R iNHL, including FL and MZL, is largely incurable, with most patients eventually experiencing disease relapse. CAR-T cell therapy represents the latest advancement in iNHL treatment paradigms, greatly improving outcomes for R/R patients. Axicabtagene ciloleucel is an autologous anti-CD19 CAR-T cell therapy containing a CD28 costimulatory domain that facilitates rapid and potent expansion to mediate targeted cytotoxicity while overcoming immune system constraints. Yescarta was approved for the treatment of adult patients with R/R FL based on the preliminary analysis of the ZUMA-5 clinical trial, a single-arm, international phase 2 trial in iNHL patients (n=104) with a median follow-up of 17.5 months and an ORR of 92% (CR rate: 74%).
Given the heterogeneity of pre-treatment tumor characteristics and the protracted clinical course in R/R indolent lymphoma, long-term follow-up analyses are particularly important. This article reports the 3-year efficacy, safety, and biomarker assessments from ZUMA-5, representing the longest follow-up analysis of CAR-T cell therapy in iNHL to date. The analysis includes exploratory evaluations of associations between clinical outcomes and baseline variables, including prior bendamustine exposure and baseline tumor burden as assessed by total metabolic tumor volume (TMTV).
Methods
The ZUMA-5 study was a multicenter, single-arm, registrational phase 2 clinical trial conducted at 17 medical centers in the United States and France. Eligibility criteria included adults aged ≥18 years with R/R iNHL, including FL (grades 1-3a) and MZL (nodal or extranodal; meeting 2016 WHO criteria), who had received ≥2 prior lines of systemic therapy, including anti-CD20 monoclonal antibody and an alkylating agent. Patients were excluded if they had received autologous stem cell transplantation (SCT) within 6 weeks, any allogeneic SCT, CD19-targeted therapy, or CAR-T cells before Yescarta. Patients with refractory disease progression within 6 months of their most recent therapy were also excluded.
Enrolled patients underwent leukapheresis, followed by lymphodepleting chemotherapy with fludarabine (30 mg/m2/day) and cyclophosphamide (500 mg/m2/day) from days -5 to -3 before infusion, and Yescarta infusion (2×106 CAR-T cells/kg). Disease response assessments were performed by investigators and reviewed by an independent radiologic review committee per Lugano criteria at designated timepoints up to 24 months of follow-up; thereafter, assessments were by investigators only.
The primary endpoint of the ZUMA-5 study was ORR. Secondary endpoints included CR, duration of response (DOR), PFS, OS, time to next treatment (TTNT), safety, and CAR-T cell levels in blood. Propensity score matching (PSM) was performed to evaluate outcomes in FL patients by prior bendamustine use, accounting for the distribution of baseline TMTV, ECOG score, FLIPI score, number of prior therapies, age, double-refractory status, and whether the last prior systemic therapy was within 12 months of leukapheresis.
Results
Patient characteristics
A total of 159 patients (127 FL, 31 MZL, 1 diffuse large B-cell lymphoma [DLBCL]) were enrolled and underwent leukapheresis, including 6 additional MZL patients enrolled after the 18-month data cutoff. Yescarta was successfully manufactured for all enrolled patients. Apart from the previously described screening failure, 1 patient had disease transformation, and 1 patient had a negative disease status. The patient diagnosed with DLBCL did not receive Yescarta and was withdrawn from the study. As of the March 31, 2022, data cutoff, 152 patients (124 FL and 28 MZL) received lymphodepleting chemotherapy and Yescarta.
Table 1. Baseline characteristics of all enrolled patients
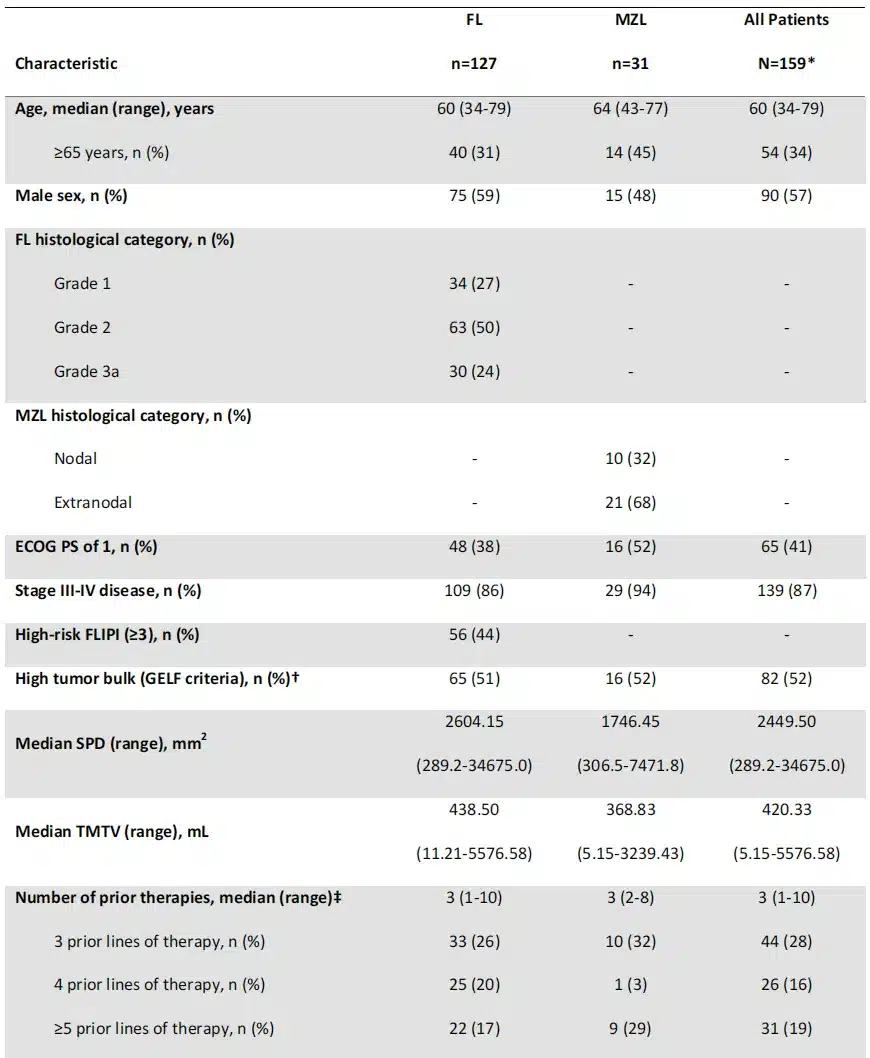
Baseline characteristics for all 159 enrolled patients are shown in Table 1. The median age was 60 (range: 34-79) years for FL patients and 64 (range: 43-77) years for MZL patients. Among FL patients, 56% had POD24, and 69% had prior bendamustine exposure. In FL patients, baseline TMTV correlated positively with tumor burden measured by the sum of the product of diameters (SPD), FLIPI score, and tumor volume by GELF criteria, but not with baseline lactate dehydrogenase levels.
Efficacy analysis in FL patients
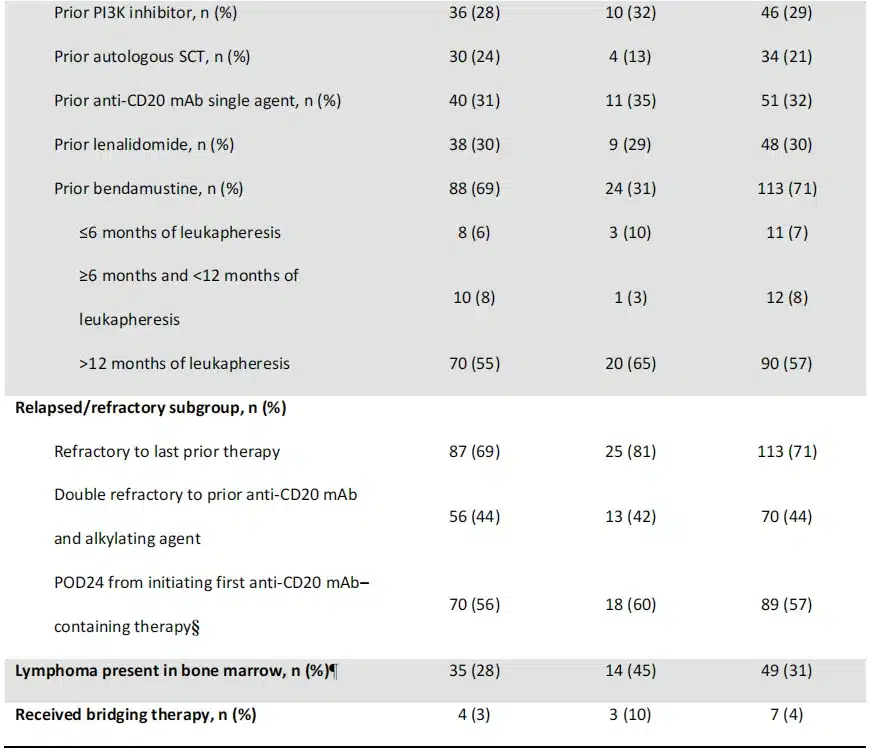
The median follow-up time for FL patients after leukapheresis was 41.7 (range: 32.7-57.4) months. Response rates assessed by investigators in FL patients were consistent with previous analyses (ORR: 94% [95% CI: 88-97]; CR rate, 79%; Table 2). Thirty-two patients initially in partial response (PR) or stable disease converted to CR. The median DOR in FL patients was 38.6 months (Table 2). The median DOR was not reached in CR patients, and was 4.9 months in PR patients. At data cutoff, 53% of FL patients (67/127) were in ongoing response; 51% (65/127) were in ongoing CR. Of those achieving CR (n=100), 65% were in ongoing response at data cutoff. Consistent with previous reports, all 13 FL patients retreated with Yescarta achieved a response (CR rate: 69%; PR rate: 31%). With a median follow-up of 23 months after retreatment, the median DOR was 5.0 months, and 46% of patients were in ongoing response at data cutoff.
Table 2. Best response by investigator assessment of all enrolled patients at 3-year analysis
For FL patients, the median PFS was 40.2 months, with an estimated 36-month PFS rate of 54% (Figure 1). After receiving leukapheresis for >24 months, there were 2 disease progression events and 10 deaths. In the competing risk analysis for lymphoma-specific PFS in FL patients, there were 41 (32%) disease progression or death events due to lymphoma, lymphodepleting chemotherapy, or Yescarta, of which 38 were disease progression events (Figure 2). The 36-month cumulative incidence of progression or lymphoma-specific death was 35%. A total of 12 patients (9%) experienced a competing risk event (progression or death unrelated to study treatment), with most occurring after the 24-month timepoint. The 36-month cumulative incidence of competing risk was 11% (Figure 2). In the analysis of the cumulative incidence of disease progression or lymphoma-related death, with non-lymphoma-related deaths (including deaths due to study treatment) considered a competing risk, the 36-month cumulative incidence of progression was 32% (no patients had died of lymphoma before progression). The 36-month cumulative incidence of competing risk was 14%. The median PFS was 40.2 months for patients with POD24 (n=70) and not reached for those without POD24 (n=41). Regardless of other high-risk baseline features, the estimated 36-month PFS rates were generally consistent in FL patients.

Figure 1. Kaplan-Meier estimates of (A) progression-free survival (PFS), (B) overall survival (OS), and (C) time to next treatment (TTNT) as assessed by the investigator in 157 enrolled iNHL patients by disease type.

Figure 2. Lymphoma-specific survival outcomes in follicular lymphoma patients by cumulative incidence and competing risks. (A) Competing risk lymphoma-specific PFS cumulative incidence; (B) Competing risk lymphoma-specific OS cumulative incidence.
Compared to FL patients who did not receive bendamustine treatment, the 36-month PFS rate was numerically lower in FL patients previously treated with bendamustine; patients who received bendamustine ≤6 months before leukapheresis had a numerically shorter PFS, although the small number of patients in this group may have limited the comparison (Figure 3).
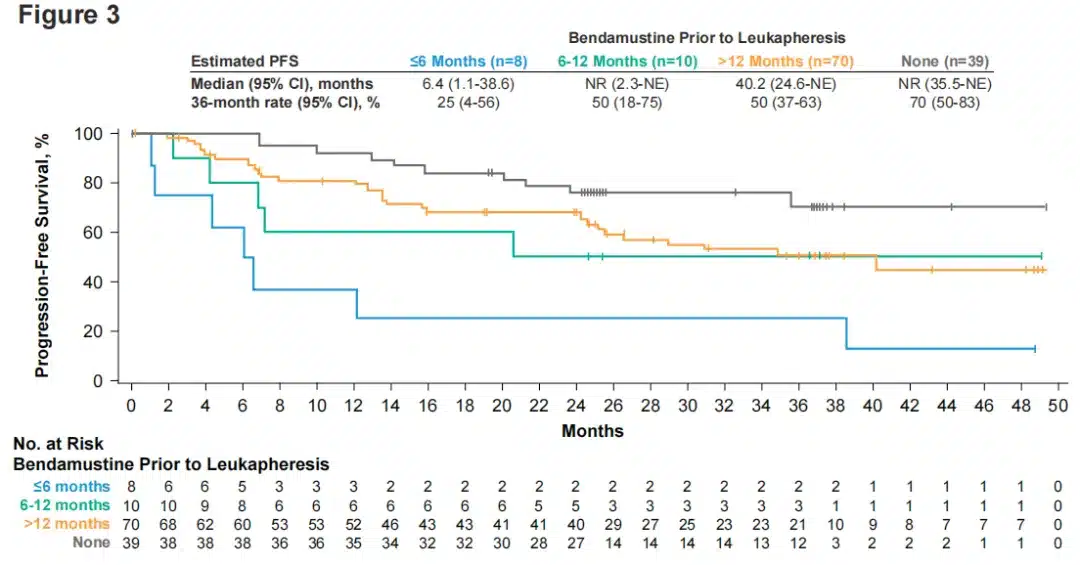
Figure 3. PFS in FL patients by time of bendamustine use before Yescarta infusion
The median OS was not reached in FL patients, with an estimated 36-month OS rate of 76% (Figure 1). The median TTNT was also not reached, with an estimated 36-month event-free rate of 60% (Figure 1). The competing risk assessment for lymphoma-specific OS showed that 16 patients (13%) died of lymphoma, lymphodepleting chemotherapy, or Yescarta. Six patients (13%) experienced a competing risk event (death from other causes). The 36-month cumulative incidence of lymphoma-specific death was 13% (the 36-month cumulative incidence of competing risk was 12%; Figure 2).
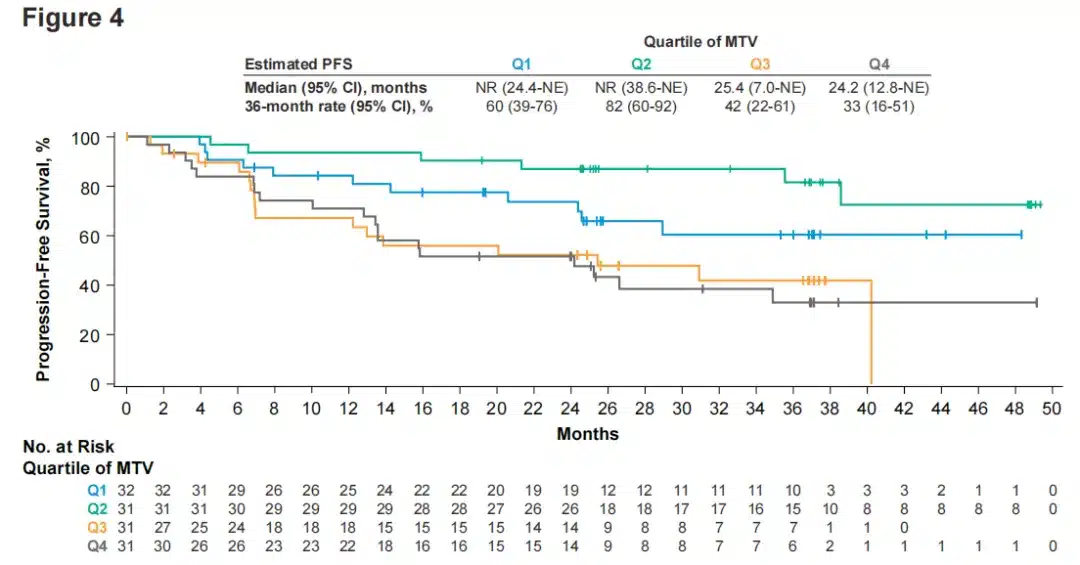
Figure 4. PFS in FL patients by baseline total metabolic tumor volume quartiles.
In the efficacy outcomes assessed by baseline TMTV in evaluable FL patients (n=125), patients with relatively lower baseline TMTV had longer DOR and PFS, below the historically reported threshold of 510 mL and below the median and quartiles observed in the study (Figure 4).
Efficacy Analysis in MZL Patients
The median follow-up time after leukapheresis for MZL patients was 31.8 (range: 8.3-52.3) months. The investigator-assessed ORR in enrolled MZL patients was 77% (95% CI: 59-90), with a CR rate of 65% (Table 2). Twelve patients converted from an initial PR or stable disease to CR. The median DOR was not reached in all MZL patients, with 52% (16/31) remaining in continued response at the data cutoff (Table 2).
The median PFS, OS, and TTNT were not reached in MZL patients, with 24-month estimates of 56%, 74%, and 53%, respectively (Figure 1). The 24-month PFS estimates were generally consistent across high-risk subgroups. No association between baseline TMTV and efficacy outcomes was observed in MZL patients, which may be due to the small number of patients with this disease type in the study.
Safety
No new safety signals were observed in iNHL-treated patients since the 18-month analysis (Table 3). AEs occurring after the 18-month analysis (data cutoff, September 14, 2020) were mainly in recently enrolled MZL patients and included 1 grade 3 neurological event, 2 grade 3-4 infections, and 5 grade 3-4 cytopenias. Since the 18-month analysis, 15 patients (10%; 11 FL and 4 MZL) experienced serious AEs, 6 of which were considered related to Yescarta (3 FL, 3 MZL; Table 3). No new cases of grade ≥3 hypogammaglobulinemia occurred after the primary analysis data cutoff (March 12, 2020). Overall, 50 iNHL patients (33%) received immunoglobulin therapy during the study. Eighteen patients developed a second primary malignancy (Table 4). No Yescarta-related second primary malignancies, tumor lysis syndrome, or replication-competent retrovirus events occurred at any time during the study.
Table 3. Summary of AEs occurring in treated patients after the 18-month analysis.
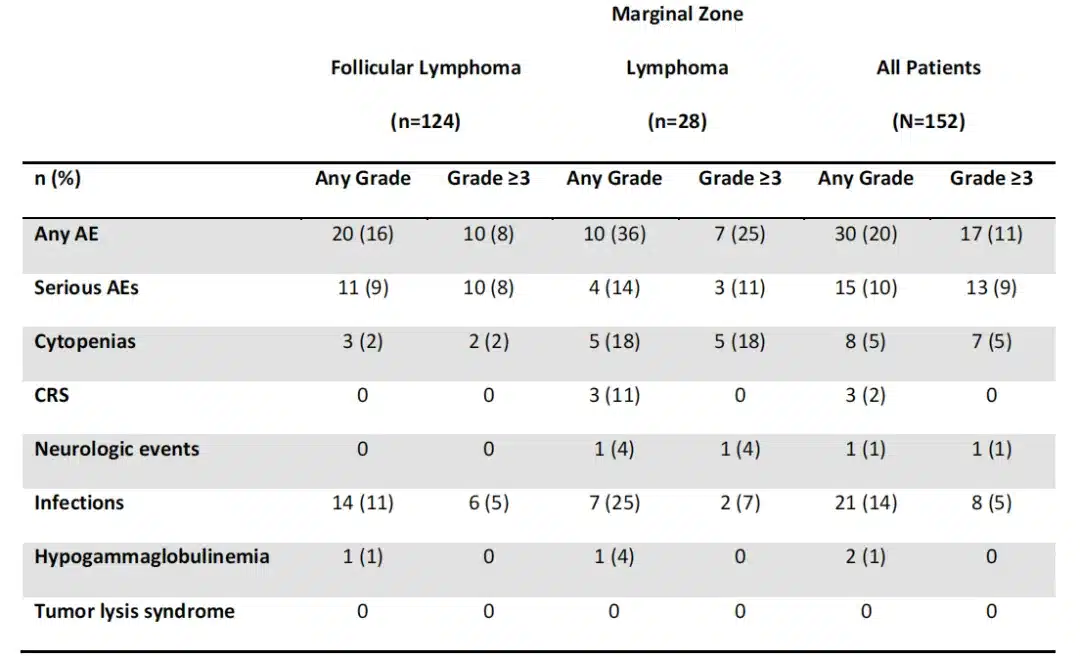
Table 4. Second primary malignancies.

Among iNHL patients (n=51) with any grade ≥3 cytopenias occurring ≥30 days after infusion, 5 had cytopenias occurring within 12 months of infusion, and 4 had cytopenias occurring within 24 months of infusion. No association was observed between baseline TMTV and grade ≥3 cytokine release syndrome or neurological events, which may be due to the low incidence of grade ≥3 toxicities. A total of 39 patients died in the ZUMA-5 study, 19 of which were assessed by the investigator as lymphoma-related (15 due to potential lymphoma complications and 4 due to study treatment-related AEs in FL patients). Among treated patients, 8 deaths were due to AEs, and 5 patients died from second primary malignancies (unrelated to Yescarta).
Study Conclusions
Overall, the 3-year long-term data from the ZUMA-5 study demonstrate durable, lasting clinical benefit with Yescarta in indolent lymphoma patients, with a manageable long-term safety profile. A substantial proportion of R/R iNHL patients remain progression-free, warranting further follow-up of survival to evaluate the curative potential of CAR-T cell therapy for this disease.
Content Source:拓麦
