Ciltacabtagene Autoleucel FDA Approval For The Treatment Of Relapsed/Refractory Multiple Myeloma
Ciltacabtagene Autoleucel FDA Approval For The Treatment Of Relapsed/Refractory Multiple Myeloma
Cittacatagene Autoleucel approved for launch in the United States
On February 28, 2022, the U.S. FDA approved Ciltacabtagene Autoleucel (cilta-cel), a CAR-T cell therapy targeting B-cell maturation antigen (BCMA), for the treatment of patients with relapsed/refractory multiple myeloma (r/r MM). Notably, this is the first China-developed CAR-T cell therapy approved by the FDA. Furthermore, this approval of the CAR-T cell product marks a new milestone and breakthrough for the internationalization of innovative drugs from China, following the approval of Zanubrutinib by Beigene, Ltd.
Ciltacabtagene Autoleucel is a differentiated chimeric antigen receptor T-cell (CAR-T) therapy, containing a 4-1BB costimulatory domain and two antibody domains targeting BCMA. The product can genetically modify the patient’s T cells to express the chimeric antigen receptor (CAR), enabling them to recognize and kill BCMA-expressing cells. BCMA is primarily expressed on the surface of malignant multiple myeloma B cells, late-stage B cells, and plasma cells.
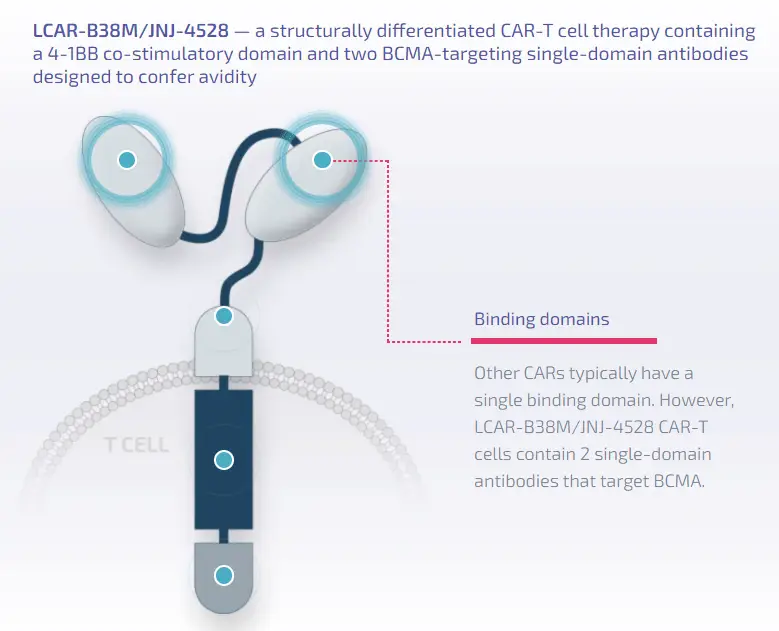
Image Description: Structural features of Ciltacabtagene Autoleucel
Introduction to Ciltacabtagene Autoleucel
Trade Name: Carvykti
Generic Name: Ciltacabtagene Autoleucel
Code: JNJ-4528/LCAR-B38M
Target: BCMA
First Approval in the U.S.: 2022
First Approval in China: Not yet approved
Approved Indication: Multiple Myeloma (r/r MM)
Clinical Data Of Ciltacabtagene Autoleucel
The approval was based on the results from the Phase Ib/II CARTITUDE-1 trial (NCT03548207), in which 97 patients received Ciltacabtagene Autoleucel treatment. The patient population characteristics included: 58.8% male; median age of 61 years; median of 6 prior lines of therapy, with 66% having received at least 5 prior therapies. Most patients (91.9%) had tumor BCMA expression on at least 50% of cells.
The primary endpoint for Phase Ib was the incidence and severity of adverse events (AEs). The primary endpoint for Phase II was the overall response rate (ORR), with secondary endpoints including stringent complete response (sCR), complete response (CR), very good partial response (VGPR), duration of response (DOR), minimal residual disease (MRD) negativity rate, progression-free survival (PFS), and overall survival (OS). Pharmacokinetics and pharmacodynamic markers were also evaluated.
According to data presented at the 63rd American Society for Hematology (ASH) Annual Meeting and Exposition, after approximately 2 years of follow-up, the ORR was 98%; 83% of patients treated with Ciltacabtagene Autoleucel achieved sCR, higher than the 67% sCR rate reported at a median follow-up of 1 year; 95% of patients achieved VGPR. The median PFS and median OS were not reached, but the 2-year PFS rate was 61%, and the 2-year OS rate was 74%.
Among the 61 evaluable patients for MRD, 92% were MRD-negative. The 2-year PFS rate for patients with MRD negativity sustained ≥6 months was 91%, and for those with MRD negativity sustained ≥12 months, the 2-year PFS rate was 100%.
Additionally, in this trial, the median time to first response was 1 month; the median time to best response was 2.6 months; and the median time to CR or better was 2.9 months.
Security Of Ciltacabtagene Autoleucel
Regarding safety, long-term follow-up data did not reveal new safety signals, and the safety profile of Ciltacabtagene Autoleucel was consistent across subgroups with the overall population. Furthermore, no new events of neurological toxicity or movement and neurocognitive treatment-emergent adverse events associated with Ciltacabtagene Autoleucel were reported.
The 18-month follow-up data presented at the 2021 ASCO meeting showed that the most common hematological adverse events observed included neutropenia (96%), anemia (81%), thrombocytopenia (79%), leukopenia (62%), and lymphopenia (53%).
Conclusion
The updated trial data demonstrate that a single infusion of Ciltacabtagene Autoleucel induces deep and durable responses in heavily pretreated patients with relapsed/refractory multiple myeloma, with the potential to improve long-term survival in this patient population.
Reference Source:https://www.onclive.com
Content Source:网罗全球好药
