Myeloma in chinese: Case Study of Relapsed/Refractory Multiple Myeloma
Myeloma in chinese: Case Study of Relapsed/Refractory Multiple Myeloma
Preface
Multiple myeloma (MM) is a malignant disease characterized by clonal proliferation of abnormal plasma cells. In many countries, it is the second most common hematological malignancy. It predominantly affects the elderly population and remains an incurable hematological malignancy to date. Most MM patients may experience multiple relapses, with each subsequent relapse exhibiting stronger drug resistance, especially after third-line or higher treatment. These patients generally have a poorer prognosis and a significant need for alternative therapies to overcome drug resistance. Daratumumab (Dara), the first fully human anti-CD38 monoclonal antibody globally, possesses a unique dual mechanism of action, directly killing myeloma cells and activating the immune system, thereby offering durable and deep remission for MM patients. In recent years, it has demonstrated promising prospects in relapsed/refractory (R/R) MM patients. In this case, we present a patient with R/R MM who experienced disease relapse after multiple lines of treatment with PAD, PCD, Pd, and VTD-PACE regimens. Subsequently, the DVD regimen in combination with chemotherapy yielded a significant response.
Patient Overview
Basic Information: Male, 50 years old.
Present Illness: The patient was admitted to the emergency department on October 16, 2017, due to dizziness, nausea, and lower back pain for one week.
Past Medical History: Hypertension for 3 years, treated with oral Nifedipine Sustained-Release Tablets, blood pressure controlled; history of lumbar disc herniation, no medication.
Physical Examination: Obese body type, clear sensorium, poor mental state, transported to the ward by stretcher, no rash or bleeding spots on the skin and mucous membranes, no palpable enlarged superficial lymph nodes, no sternal tenderness, normal heart and lung auscultation, liver and spleen not palpable below the costal margin, mild edema in both lower extremities.
Auxiliary Examinations
Routine Blood Tests: WBC 13.66×109/L, ANC 12.32×109/L, Hb 156g/L, PLT 162×109/L.
Tumor Markers: Normal immunoglobulins IgG/A/M, Immunofixation Electrophoresis: LAM type M-protein in serum, Urine Protein Qualitative: Positive, Serum Free Light Chain κ: 9.79mg/L, Urine Free Light Chain κ: 70.2mg/L.
Blood Chemistry: Albumin 36.9 g/L, Globulin 27.8 g/L, β2-Microglobulin 6.56 mg/L, Creatinine 179.5 umol/L, Uric Acid 1017 umol/L, LDH 257 U/L, Calcium 4.8 mmol/L, Carbon Dioxide 38.5 mmol/L, ESR 72 mm/h.
Urinalysis: Urine Protein +-; Early Renal Impairment: Urine Microalbumin 2384 mg/L;24-hour Urine Protein Quantitation: 5980 mg/24h.
Imaging Examinations
Skull and Pelvic X-rays: Multiple variably sized cystic low-density lesions seen in the skull and pelvis, suggestive of multiple myeloma.
Chest and Abdominal CT: Multiple lytic lesions seen in the thoracic and lumbar vertebrae, sternum, ribs, and pelvic bones, consistent with multiple myeloma; lower lung inflammation, minimal pleural effusion.
Bone Scan: Uniform and symmetrical radiotracer distribution in the skull, sternum, clavicles, scapulae, ribs, and long bones of the extremities; a radiotracer defect seen in the right pelvic bone, and multiple areas of increased radiotracer uptake in the thoracic vertebrae, suggestive of multiple myeloma.
Bone Marrow Examination
Bone Marrow Morphology: Active marrow proliferation, plasma cells accounting for approximately 28.5%.
Bone Marrow Biopsy + Immunohistochemistry: Expressing CD38, CD138, and lambda, suggestive of MM.
MM-FISH: Negative
Karyotype: 46, XY [20]
Diagnosis
– Multiple Myeloma (LAM type, DS and ISS stage III B)
– Renal Impairment
– Hypercalcemia
– Hypertension (Grade 3, High Risk)
Treatment Course
First Phase (2017.10.18 ~ 2018.12.5)
Pre-chemotherapy: Hydration, furosemide diuresis, urine alkalinization, treatment for hypercalcemia.
Zoledronic Acid 4mg IV drip 1d; Salmon Calcitonin 500IU IV drip 2d (Follow-up Blood Chemistry: Calcium decreased from 4.8 mmol/L to 2.5 mmol/L, Uric Acid decreased from 1017 umol/L to 391 umol/L).
PAD regimen combined chemotherapy for 9 cycles (Bortezomib 1.3 mg/m2 on days 1, 4, 8, 11; Epirubicin 30 mg on days 1-4; Dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, 12).
Second Phase (2018.12.5 ~ 2019.2.19)
PCD regimen combined chemotherapy for 2 cycles (Bortezomib 1.3 mg/m2 on days 1, 4, 8, 11; Cyclophosphamide 350 mg/m2 on days 1, 8, 15, 22; Dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, 12). Response evaluation: CR.
Autologous stem cell transplantation in April 2019, followed by monthly Pd (Pomalidomide + Dexamethasone) consolidation therapy.
Third Phase (2019.12.19 ~ 2021.5-5)
Bone Marrow Morphology: CR.
Minimal Residual Disease: Negative.
Abnormal Immunoglobulin Diagnosis: Immunofixation Electrophoresis: Weakly positive LAM type M-protein, Urine Protein Qualitative: Weakly positive.
PCD regimen combined chemotherapy for 7 cycles (Bortezomib 1.3 mg/m2 on days 1, 8, 15, 22; Cyclophosphamide 0.3 g/m2 on days 1, 8, 15, 22; Dexamethasone 40 mg on days 1, 8, 15, 22).
Fourth Phase (2021.5.5-2022.7.1)
Abnormal Immunoglobulin Diagnosis:
Immunofixation Electrophoresis: LAM type M-protein.
Urine Protein Qualitative: Positive.
Immunophenotyping: Abnormal CD45dimCD38bright cells accounting for 0.43% of nucleated cells, expressing CD38 and clambda, partially expressing CD56, and not expressing CD19, suggestive of residual multiple myeloma cells.
VTD-PACE regimen combined chemotherapy for 5 cycles (Bortezomib 1.3 mg/m2; Dexamethasone 30 mg on days 1-4; Epirubicin 20 mg on days 1-4; Cisplatin 20 mg on days 1-4; Cyclophosphamide 0.8 g on days 1-4; VP-16 80 mg on days 1-4; Thalidomide 100 mg oral qn).
Outpatient maintenance therapy with Pomalidomide + Dexamethasone.
Fourth Relapse on 2022.7.3:
Follow-up Blood Tests: WBC 4.32×109/L, Hemoglobin 106g/L, Platelets 38×109/L.
ESR: 92 mm/h; β2-Microglobulin 11.7 mg/L.
Blood Chemistry: Blood Urea Nitrogen 32.0 mmol/L, Creatinine 355.3 umol/L, Uric Acid 851 umol/L.
Calcium: 3.11 mmol/L.
Bone Marrow Smear: Decreased marrow proliferation, myeloma cells accounting for approximately 23.5%, binucleated myeloma cells observed; Karyotype: 46, XY [7].
MM-FISH: Negative.
Fifth Phase (2022.07.04 ~ 2022.07.25)
On 2022.07.04, the patient received 1 cycle of DVD regimen combined chemotherapy (Daratumumab 1000 mg qw, Bortezomib 1.3 mg/m2 on days 1, 2, 8, 9, 15, 16, Dexamethasone 20 mg on days 1, 2, 8, 9, 15, 16, 22, 23).
After one cycle of DVD regimen combined chemotherapy, the patient’s chest tightness significantly improved, and creatinine and NT-proBNP levels markedly decreased.
On July 26, 2022, the patient was discharged due to clinical improvement but did not return for subsequent scheduled treatment for unknown reasons.
Response Evaluation
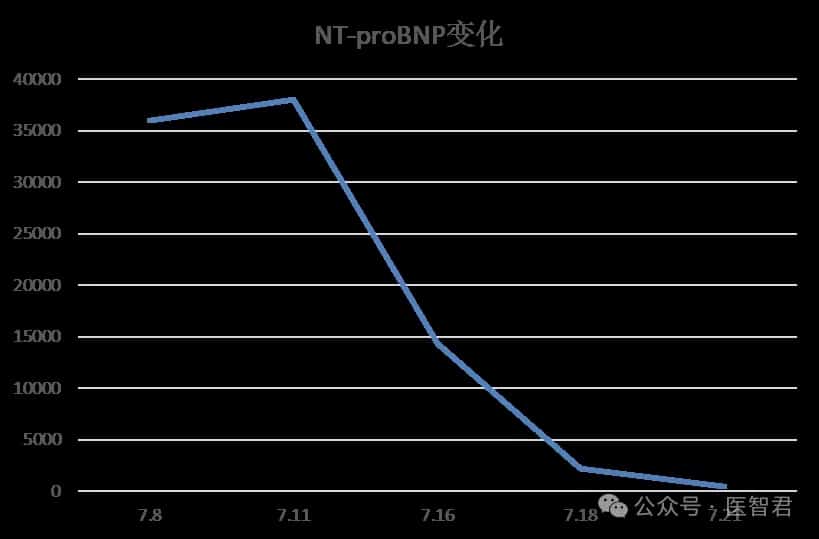
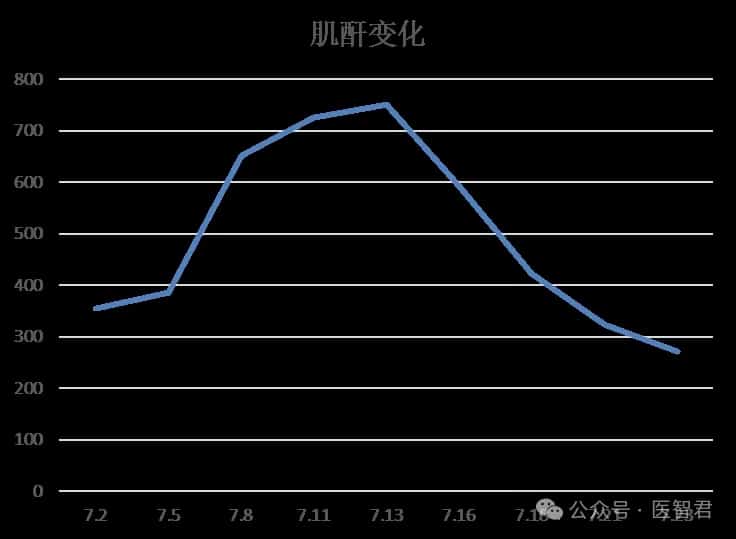
Case Summary and Treatment Reflections
To date, multiple myeloma remains an incurable hematological malignancy, but the development and clinical application of new drugs have led to improved treatment outcomes and prolonged survival for MM patients. For patients with relapsed/refractory (R/R) MM, various treatment options are currently available, including proteasome inhibitors such as Bortezomib and Carfilzomib; immunomodulatory agents including Lenalidomide and Pomalidomide; and antibody therapies such as anti-CD38 monoclonal antibodies.
This case describes a middle-aged male patient who was admitted to the hospital due to dizziness, nausea, and lower back pain for one week. After undergoing bone marrow aspiration, imaging studies, and related examinations, he was diagnosed with multiple myeloma. The patient received multiple combination chemotherapy regimens, including PAD, Pd, PCD, and VTD-PACE, leading to initial clinical improvement, but subsequently experienced multiple relapses. According to the Chinese Guidelines for Diagnosis and Treatment of Multiple Myeloma (2022 Edition), Daratumumab-based combination regimens (DVd, DKd, DPd) are recommended as treatment options for patients with relapsed multiple myeloma. Consequently, the patient was treated with the Daratumumab-based DVD regimen, which provided a certain degree of efficacy. Unfortunately, for unknown reasons, the patient did not return for scheduled treatment after discharge.
References:
中国抗癌协会血液肿瘤专业委员会, 中华医学会血液学分会. 中国多发性骨髓瘤骨病诊治指南(2022年版) [J] . 中华血液学杂志, 2022, 43(12) : 979-985. DOI: 10.3760/cma.j.issn.0253-2727.2022.12.002.
Content Source:医智君
