Multiple Myeloma Chinese:High-Risk Multiple Myeloma Patient Achieves Cancer Cell Clearance in the First Month After CAR-T Therapy
Multiple Myeloma Chinese:High-Risk Multiple Myeloma Patient Achieves Cancer Cell Clearance in the First Month After CAR-T Therapy
“Congratulations, you can be discharged. The treatment efficacy evaluation is excellent, and you have achieved a Complete Response (CR) with no tumor cells remaining in your body.” When Mr. Zhang (pseudonym), a resident of Yunnan Province, heard these words from Dr. Yang, he felt a great sense of relief and disbelief. Just a month ago, he was a high-risk multiple myeloma patient who couldn’t even get out of bed. But under the dedicated care of the doctors from the Department of Hematology at the First Affiliated Hospital of Zhejiang University School of Medicine, a miracle had occurred…
Rib Pain Three Years Ago Turned Out to Be Bone-Eating Cancer
Mr. Zhang (pseudonym), over 50 years old this year, experienced unexplained dull pain in his left chest three years ago. After an examination at a local hospital, he was told it was a fracture in his left rib and was advised to rest at home. As a result, Mr. Zhang did not pay much attention or seek timely treatment. However, as the pain worsened and became increasingly unbearable, Mr. Zhang visited a major local hospital for a comprehensive examination. Unexpectedly, what was initially thought to be a rib fracture turned out to be a malignant blood cancer – multiple myeloma.
Faced with this diagnosis, Mr. Zhang and his family found it difficult to accept and were caught off guard. Multiple myeloma? What kind of disease is this, and is it curable? After some research, they learned that multiple myeloma (MM) is a clonal plasma cell proliferative disorder and the second most common malignant tumor of the blood system, which currently has no cure. Furthermore, because the tumor cells can cause bone damage, pain, and even fractures and paralysis, it is also known as “bone-eating cancer.”
This reality brought a sense of helplessness to Mr. Zhang, who was in the prime of his life and the breadwinner of his family. However, the love and support from his family motivated him to actively seek treatment and fight for his life.
Chemotherapy + Autologous Transplant, Traditional Therapies Failed to Eradicate the Tumor
After being diagnosed with multiple myeloma, Mr. Zhang (pseudonym), like many patients, embarked on a long and painful journey of chemotherapy. Some of the chemotherapy drug names are etched in Mr. Zhang’s memory, as he experienced the debilitating side effects for the first time. Subsequently, on the recommendation of his doctors, Mr. Zhang underwent an autologous stem cell transplant and achieved remission during the treatment efficacy evaluation. At this point, Mr. Zhang’s condition was initially brought under control.
However, the respite was short-lived, and the myeloma cells began to reactivate in Mr. Zhang’s body. In 2022, Mr. Zhang visited a local hospital due to physical discomfort and was informed that his cancer had relapsed. “Prone to relapse” is a major characteristic of multiple myeloma, but the speed at which it relapsed caught Mr. Zhang off guard.
After the first relapse, Mr. Zhang started receiving immunotherapy, a common treatment for multiple myeloma. However, around half a month into the treatment, Mr. Zhang’s platelet count dropped significantly below the normal range, posing difficulties for subsequent treatment.
By the end of 2022, Mr. Zhang’s condition took a turn for the worse, and the local hospital had no better treatment options, recommending him to seek treatment elsewhere. After some recommendations, Mr. Zhang traveled thousands of miles to the Department of Hematology at the First Affiliated Hospital of Zhejiang University School of Medicine (hereinafter referred to as “ZY Hospital”) for treatment.
Upon admission, the medical team at ZY Hospital conducted a more comprehensive examination and multidisciplinary consultation for Mr. Zhang, formulating a multi-drug combination treatment plan. Subsequently, they repeatedly adjusted the medication regimen, virtually exhausting all three major traditional drug classes for treating multiple myeloma. However, his condition remained uncontrolled, and the treatment efficacy was unsatisfactory. This is a common predicament faced by multiple myeloma patients with multi-line relapse and drug resistance, severely threatening their lives.
A Ray of Hope: CAR-T Approval Gives Patients a Second Chance at Life
Based on Mr. Zhang’s (pseudonym) various test results, he was classified as a high-risk multiple myeloma patient. After multiple rounds of treatment, his bone marrow hematopoietic function had significantly declined, making treatment extremely difficult with limited options.
In this dire situation, after a comprehensive evaluation by Professors Jin Jie and Yang Min’s team at the First Affiliated Hospital of Zhejiang University School of Medicine, they determined that CAR-T cell therapy was Mr. Zhang’s only possible and promising treatment option. Fortunately, on June 30, 2023, China’s first fully human CAR-T cell therapy for treating multiple myeloma was approved for marketing. Research has shown that this cell therapy can significantly improve the remission rate and quality of life for patients with relapsed or refractory multiple myeloma. At the same time, this was the first case in Zhejiang Province to receive commercialized CAR-T treatment for multiple myeloma.
Thus, a race against time, a struggle to save a life, and a collaborative effort involving medical staff, family members, and pharmaceutical companies in the entire CAR-T treatment process began…
Step One: The Well-Organized Leukapheresis Process
CAR-T therapy primarily involves collecting the patient’s T cells, genetically modifying them in vitro to enhance their ability to specifically recognize and target tumor cells. Subsequently, the large number of “armed” T cells (i.e., CAR-T cells) are reinfused into the patient to precisely kill the tumor cells. Therefore, leukapheresis is the first step in determining whether CAR-T treatment can be administered.
Mr. Zhang lay quietly beside the leukapheresis machine, while the medical staff around him worked diligently yet systematically. Previously, Mr. Zhang’s low platelet count had posed difficulties for leukapheresis. However, after thorough communication and understanding with Mr. Zhang’s family, the medical staff administered platelet transfusions simultaneously with leukapheresis, allowing them to complete the collection process after several hours.
Step Two: Time-Critical Sample Transportation
CAR-T therapy is a personalized treatment process, and its production must strictly comply with Good Manufacturing Practice (GMP) regulations. Therefore, the leukapheresis samples must be transported by professional logistics personnel from the manufacturing company to the production site as quickly as possible, maintaining the appropriate cold chain temperature range (2-8°C) throughout the journey, to enter the commercial CAR-T production process.
Step Three: The Crucial Lymphodepletion Plan
Patients receiving CAR-T therapy generally have to wait around 20 days after leukapheresis for the CAR-T cells to be produced before they can be reinfused. During this waiting period, an essential step is to perform lymphodepletion pretreatment on the patient to create a favorable bodily environment for the reinfusion. During this time, Mr. Zhang’s physical condition was unstable, and his disease had progressed. The experienced medical team at ZY Hospital administered a series of bridging treatments to help restore some of Mr. Zhang’s body indicators, allowing him to undergo the lymphodepletion plan and successfully complete this stage.
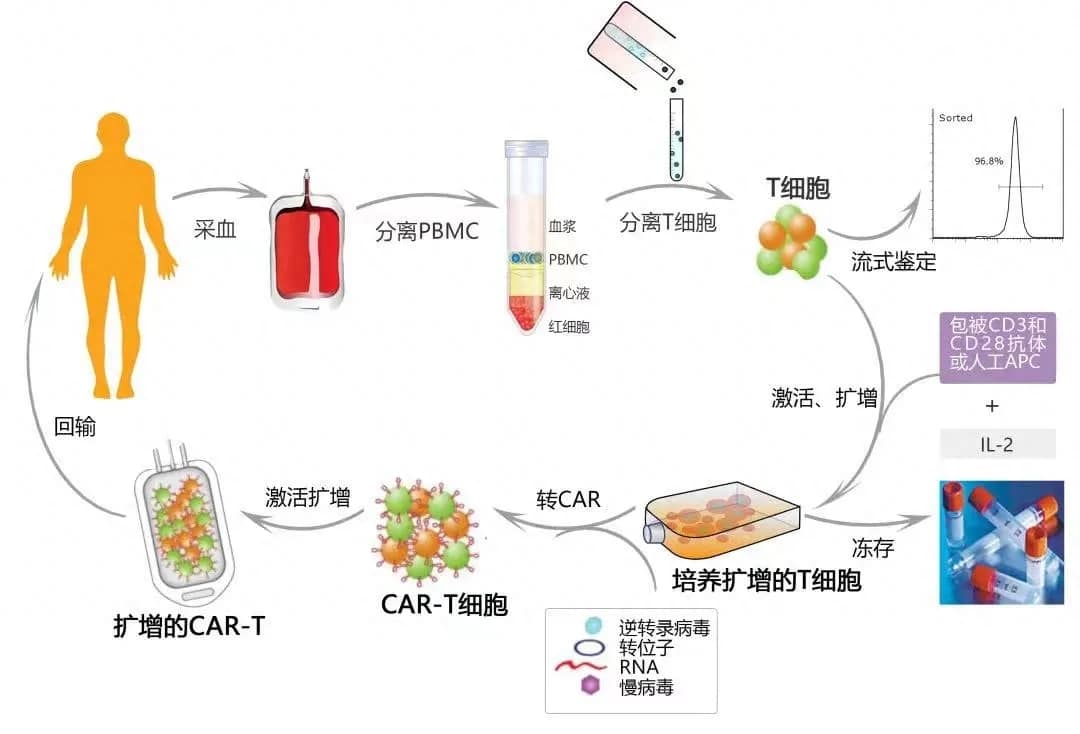
Step Four: The Memorable Successful Reinfusion
After passing through over a hundred high-standard, strict quality control steps in the commercial CAR-T production process, Mr. Zhang’s precious peripheral blood cells were successfully transformed into his personalized CAR-T cells, potentially life-saving. However, more challenges awaited. Mr. Zhang’s fever, caused by multiple factors, affected the reinfusion plan. But with the effective management of the ZY Hospital medical staff, Mr. Zhang’s fever was brought under control, and the medical team deemed him fit for CAR-T cell reinfusion. Subsequent tests showed that the CAR-T cells were continuously expanding and proliferating in Mr. Zhang’s body, progressing as expected. At the same time, the ZY Hospital medical team was well-prepared to manage potential adverse events like Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS). Through effective management, Mr. Zhang safely navigated the post-reinfusion adverse event phase.
Step Five: Witnessing the Miracle in the Efficacy Evaluation
The effectiveness of a drug must be determined by “convincing evidence,” and the treatment response rate can quickly and intuitively reflect this. For instance, a durable and deep complete response (CR) is what every cancer patient and clinician hopes for.
21 days after Mr. Zhang’s CAR-T cell infusion, tests showed that the CAR-T cells continued to expand and increase in proportion in his body. Simultaneously, his free light chain levels decreased significantly, the M protein concentration dropped, and pathological examination revealed no abnormal plasma cells. Following a next-generation flow cytometry (NGF) assessment for minimal residual disease (MRD), his treatment response was evaluated as MRD negative.
Recently, the efficacy evaluation for Mr. Zhang has achieved a complete response (CR) with MRD negativity. Clinically, achieving a complete response and MRD negativity indicates a more thorough clearance of cancer cells, often portending a longer and more durable survival. With this, the “Zheyi” medical team declared that Mr. Zhang could be discharged for convalescence.
Doctors’ Reflections
Professor Jin Jie: Bringing a ray of hope in the darkest hour, CAR-T therapy offers new hope for patients with relapsed or refractory multiple myeloma!
Among the approved or ongoing clinical studies of CAR-T therapy for multiple myeloma, there are different types of treatment targets. The B-cell maturation antigen (BCMA) is highly expressed in malignant plasma cells, making it an ideal target for multiple myeloma treatment. Multiple studies have shown that BCMA CAR-T can further improve the response rate and survival period for patients with relapsed or refractory multiple myeloma.
Professor Yang Min: Remarkably effective, CAR-T successfully treats multiple myeloma patients after multiple relapses!
This is the first case of a multiple myeloma patient in Zhejiang Province to receive commercial CAR-T therapy. The patient had multiple high-risk factors and poor bone marrow function and organ dysfunction after previous multi-line treatments. However, with the standardized, reasonable, and timely management by our medical staff, the understanding and support of the family members, and the full cooperation of the manufacturing company throughout the process of CAR-T cell collection, preparation, bridging therapy, lymphodepletion, and infusion, as well as post-infusion safety management, we completed the treatment through concerted efforts. In particular, this case demonstrates that even for high-risk patients facing various risks, CAR-T therapy can still be administered under reasonable and standardized clinical management, benefiting from the excellent efficacy and controllable safety profile of the fully human CAR-T product. This has added confidence to the clinical treatment of multiple myeloma patients.
References:
[1]杜文,王晓青,成娟.靶向BCMA CAR-T细胞在复发难治性多发性骨髓瘤中的治疗进展[J]. 中国肿瘤临床,2021,48(16):858-862.
[2]中华医学会血液学分会浆细胞疾病学组,中国医师协会多发性骨髓瘤专业委员会. 中国首次复发多发性骨髓瘤诊治指南(2022年版)[J]. 中华血液学杂志,2022,43(10):810-817.
[3]王莹,徐开林. CAR-T治疗多发性骨髓瘤的进展[J]. 临床内科杂志,2022,39(9):589-593.
[4]刘澎,李晶,徐天虹,等. 多发性骨髓瘤的多学科协作诊疗模式[J]. 临床血液学杂志,2021,34(7):454-457.
Content source:髓心守护关爱之家
