Successful Case of CAR-T Cell Therapy Equecabtagene Autoleucel (CT103A) Treatment in China
Successful Case of CAR-T Cell Therapy Equecabtagene Autoleucel (CT103A) Treatment in China
Multiple myeloma (MM) remains an incurable malignancy to date. In recent years, with the continuous development of chimeric antigen receptor T-cell immunotherapy (CAR-T), the outcome of MM has seen more possibilities. In August 2023, the Department of Hematology at Nanfang Hospital, Southern Medical University, admitted a case of MM. The patient had relapsed after multiple lines of prior treatment and progressed to refractory MM with extramedullary disease. Subsequently, the patient received treatment with the fully human BCMA CAR-T cell therapy idecabtagene vicleucel and achieved a complete response with the disappearance of all lesions. Here, we invited Professor Feng Ru, Professor Wei Xiaolei, and Professor Guo Xutao from the Department of Hematology, Nanfang Hospital, Southern Medical University, to share the diagnosis and treatment process of this patient and their treatment experience.
Patient History
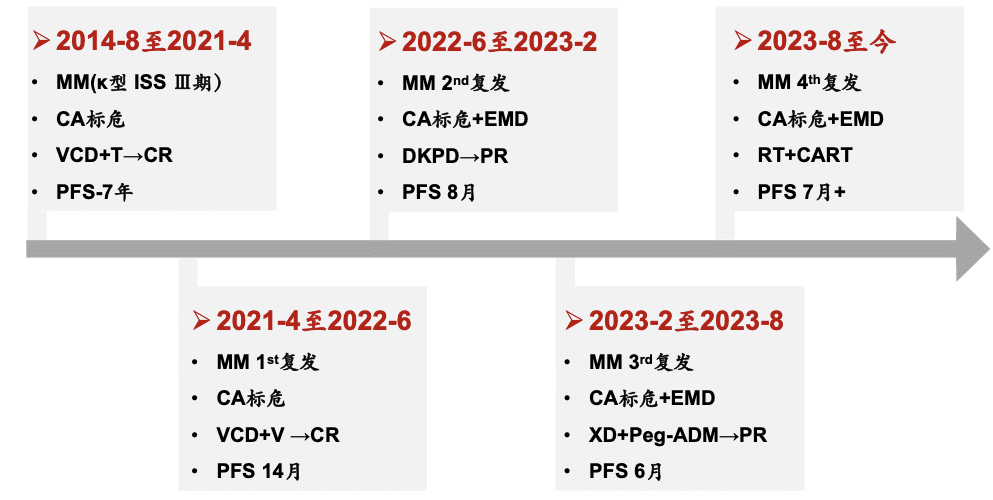
The patient was a 42-year-old female who was admitted to the hospital in August 2014 due to foamy urine for half a year and elevated creatinine for 1 month. After a comprehensive examination, she was diagnosed with multiple myeloma (kappa light chain type, ISS stage III, DS stage IIIB). The patient’s condition progressed rapidly, and her renal function deteriorated sharply, requiring regular dialysis. In August 2014, the patient started first-line treatment with VCD chemotherapy plus lenalidomide maintenance, achieving a 7-year progression-free survival (PFS). In April 2021, the patient’s lung CT scan showed bone destruction, and further examination revealed disease progression. She received second-line VCD chemotherapy plus bortezomib maintenance, achieving a 14-month PFS. In June 2022, the patient developed proptosis, and PET-CT and MRI scans suggested extramedullary plasmacytoma. Third-line and fourth-line treatments with DKPD and XD+Peg-ADM regimens, respectively, were given, but the best response achieved was only a partial response (PR), with increasingly shorter PFS durations. The treatment course is shown in the following table:
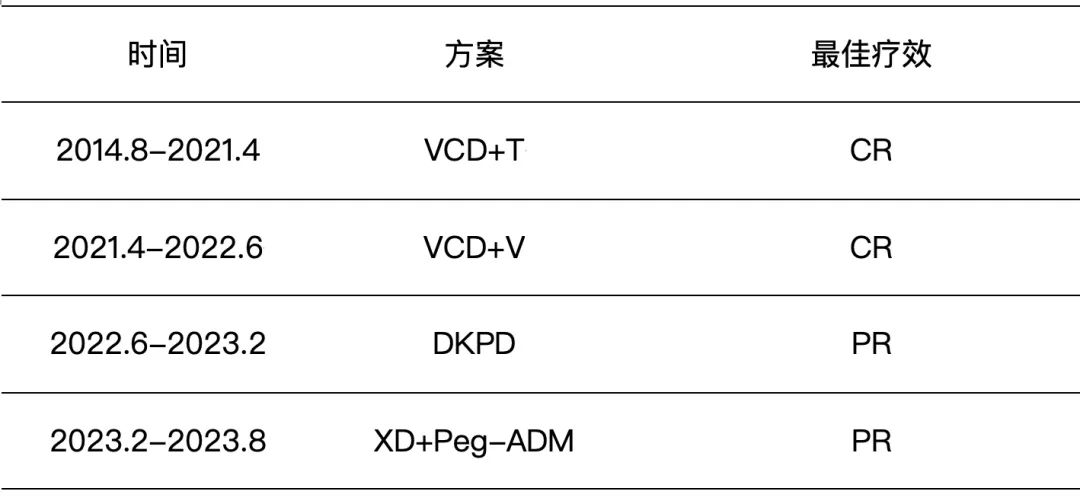
Note: VCD regimen: bortezomib, cyclophosphamide, dexamethasone; DKPD regimen: daratumumab, carfilzomib, pomalidomide, dexamethasone; XD regimen: selinexor, dexamethasone.
Over the past decade, this patient experienced multiple relapses and had exhausted nearly all available treatment options for multiple myeloma, but the disease remained difficult to control. Based on the patient’s disease characteristics and treatment history, after repeated communication with the patient and her family, Professors Feng Ru and Wei Xiaolei’s team decided to proceed with CAR-T cell therapy. In August 2023, the patient’s peripheral blood lymphocytes were collected, and CAR-T cells were prepared. During the waiting period, bridging radiotherapy was used to locally control the progression of the extramedullary plasmacytoma. In October 2023, the patient underwent FC regimen lymphodepletion preconditioning, along with entecavir for HBV reactivation prophylaxis, valacyclovir for herpes virus prophylaxis, and intravenous immunoglobulin for hypogammaglobulinemia prophylaxis. On day +8 after CAR-T cell infusion, the patient developed fever with a maximum temperature of 38.9°C, but her oxygen saturation and blood pressure remained normal, consistent with grade 1 cytokine release syndrome (CRS). After symptomatic treatment, the condition improved. The patient was discharged on day +15. The one-month evaluation after CAR-T cell infusion showed that the patient achieved a hematological complete response (CR), and the two-month evaluation showed CR for the extramedullary plasmacytoma, with negative minimal residual disease (MRD) in the bone marrow.
Treatment Experience
This patient was a young female diagnosed with kappa light chain multiple myeloma, with standard-risk cytogenetics but requiring regular dialysis, which increased the difficulty of subsequent treatment. After first-line treatment, the patient achieved 7 years of disease control, but her response duration became shorter after second-line treatment, and she developed extramedullary plasmacytoma. Nearly all available anti-myeloma drugs had been used, but the disease progression remained difficult to control.
Based on the patient’s condition and treatment history, idecabtagene vicleucel treatment was chosen for her. During this period, considering the patient’s extramedullary plasmacytoma, bridging radiotherapy was used to control the local lesions. Due to the patient’s need for regular dialysis, adjustments were made to the FC lymphodepletion regimen. After idecabtagene vicleucel infusion, the patient achieved a striking response, with hematological and extramedullary plasmacytoma CR, and negative bone marrow MRD by flow cytometry. During treatment, the patient only experienced grade 1 CRS, which improved with supportive care, and no immune effector cell-associated neurotoxicity syndrome (ICANS) occurred, demonstrating good safety. Moving forward, we will continue to follow up on the patient’s long-term response and the persistence of CAR-T cells, providing close monitoring and evaluation.
Content source:Htology血液前沿
