China’s Second CAR-T Cell Therapy Zevorcabtagene Autoleucel (CT053) Approved for Treatment of Multiple Myeloma
China’s Second CAR-T Cell Therapy Zevorcabtagene Autoleucel (CT053) Approved for Treatment of Multiple Myeloma
2024 seems to be a bumper year for cell therapy. Following the global launch of the first approved TIL cell therapy, lifileucel, the second CAR-T product in China for the treatment of multiple myeloma, Zevorcabtagene Autoleucel (赛恺泽), has finally received approval from the National Medical Products Administration (NMPA) for its new drug application to treat adult patients with relapsed or refractory multiple myeloma.
On March 1, 2024, the National Medical Products Administration officially issued a notice approving the new drug application (NDA) for Zevorcabtagene Autoleucel injection (赛恺泽®, zevor-cel, product code: CT053) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent.
About Zevorcabtagene Autoleucel
Zevorcabtagene Autoleucel is an autologous CAR-T product targeting BCMA, featuring a CAR construct with a fully human BCMA-specific single-chain variable fragment designed for lower immunogenicity and higher stability. The product received Regenerative Medicine Advanced Therapy (RMAT) and Orphan Drug designations from the U.S. FDA in 2019, as well as PRIME designation and Orphan Drug designation from the European Medicines Agency (EMA) in 2019 and 2020, respectively. It also received Breakthrough Therapy designation from the National Medical Products Administration of China in 2020.
Basis of this approval
This approval is based on an open-label, single-arm, multi-center Phase II clinical trial (LUMMICAR STUDY 1, NCT03975907) conducted in China. According to the trial results presented at the 2022 American Society of Hematology (ASH) meeting, Zevorcabtagene Autoleucel demonstrated encouraging efficacy and a favorable safety profile.
Starting from July 23, 2019, 14 patients with a median age of 54 years (range 34-62 years) received a single infusion of Zevorcabtagene Autoleucel. As of the data cutoff date (July 17, 2023), the median follow-up for survival was 37.7 months. The overall response rate was 100%, with 11 patients (78.6%) achieving complete response (CR) or stringent complete response (sCR), 2 patients (14.3%) achieving very good partial response, and 1 patient (7.1%) achieving partial response. All patients who achieved CR or better had negative minimal residual disease (MRD).
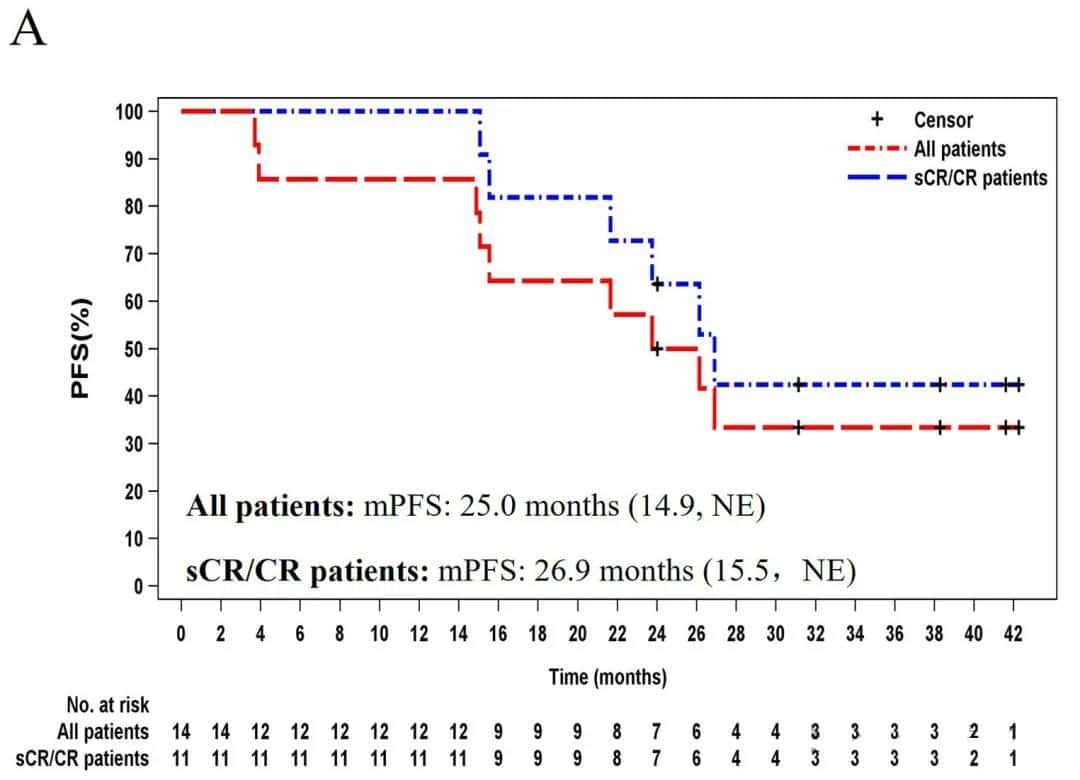
The median progression-free survival for all patients was 25.0 months, and for patients with sCR/CR, it was 26.9 months.
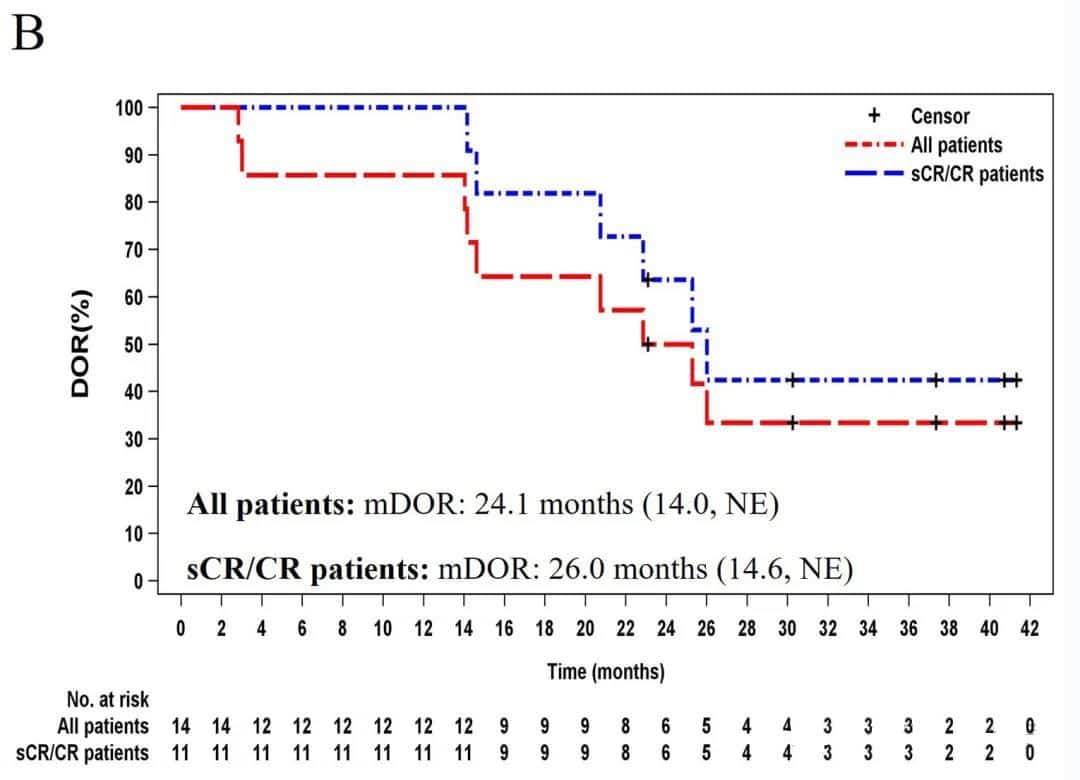
The median duration of response for all patients was 24.1 months, and for patients with sCR/CR, it was 26.0 months. At the data cutoff, 5 subjects had an ongoing response.
The study demonstrated that Zevorcabtagene Autoleucel showed deep and durable responses in patients with relapsed or refractory multiple myeloma, with an overall favorable tolerability profile.
