2024 EBMT|Latest Data Disclosure on China’s Harvesting of RRMM CAR-T Cell Therapy Equecabtagene Autoleucel (CT103a): Efficacy Unaffected by Patients’ Baseline sBCMA Plasma Levels
2024 EBMT|Latest Data Disclosure on China’s Harvesting of RRMM CAR-T Cell Therapy Equecabtagene Autoleucel (CT103a): Efficacy Unaffected by Patients’ Baseline sBCMA Plasma Levels
In recent years, BCMA-targeted CAR-T therapy as a novel treatment approach has brought new hope to patients. The 50th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT) was held from April 14-17, 2024, in Glasgow, United Kingdom. During this EBMT meeting, Professor Qiu Lugui’s team reported the latest subgroup analysis results from the FUMANBA-1 study (Abstract No. OS10-04) on B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor T (CAR-T) cell therapy for relapsed or refractory multiple myeloma (RRMM).
Soluble BCMA (sBCMA) is a biomarker of tumor burden in multiple myeloma patients. The accumulation of sBCMA may competitively bind with BCMA on myeloma cells, reducing the cytotoxic effect of BCMA-targeted therapies, including BCMA CAR-T cells, on myeloma cells and affecting the pharmacokinetics and pharmacodynamics of BCMA CAR-T cells, such as increasing CAR-T cell exhaustion and decreasing the efficacy of BCMA-targeted therapies. As reported at the 2023 ASH meeting, the efficacy of ciltacabtagene autoleucel (Cilta-cel) in the CARTITUDE-1 study was significantly impacted by high baseline sBCMA levels, resulting in decreased progression-free survival (PFS) [1]. In contrast, equecabtagene autoleucel (Equecart) was designed and developed to minimize and avoid the impact of sBCMA on its overall response rate (ORR) and PFS through the screening and selection of the single-chain variable fragment (scFv).
Abstract No.: OS10-04
Title: BASELINE SBCMA PLASMA LEVEL HAS NO IMPACT ON THE EFFICACY OF EQUECABTAGENE AUTOLEUCEL, A BCMA-CAR T CELL THERAPY IN PATIENTS WITH RRMM: SUBANALYSIS OF FUMANBA-1 TRIAL
Background
B-cell maturation antigen (BCMA) is a promising therapeutic target for multiple myeloma (MM) treatment, and soluble BCMA (sBCMA) originates from the shedding of the extracellular domain of BCMA. Serum sBCMA levels can predict patient survival and monitor disease progression; high tumor burden, R-ISS stage III, DS stage III patients, and patients with high BCMA expression tend to present with high sBCMA levels. On the other hand, reports suggest that sBCMA fragments can inhibit the function of BCMA CAR-T cells. Sufficient sBCMA can accumulate in the bone marrow of MM patients, inhibiting the recognition of tumor cells by BCMA CAR-T cells and potentially limiting the efficacy of BCMA-targeted CAR-T therapies and increasing CAR-T cell exhaustion (Image 1) [1].
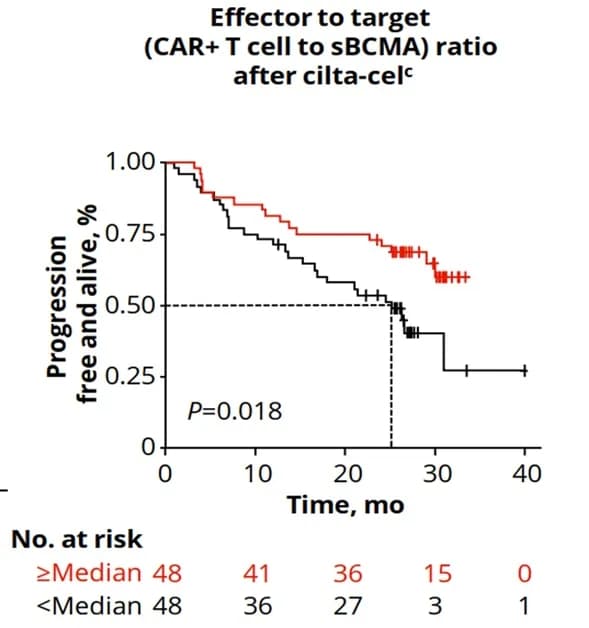
Image 1: The CARTITUDE-1 study showed that high sBCMA levels were associated with lower PFS (patients with a lower CAR-T cell to sBCMA ratio had significantly reduced PFS; high baseline sBCMA levels were significantly associated with reduced PFS, P=0.0098).
Therefore, in the design and development process of equecabtagene autoleucel (CT103A), the first marketed BCMA CAR-T product in China, IASO Biotechnology selected a suitable scFv to minimize the competitive inhibition of sBCMA binding to cell surface BCMA (Image 2) [2].
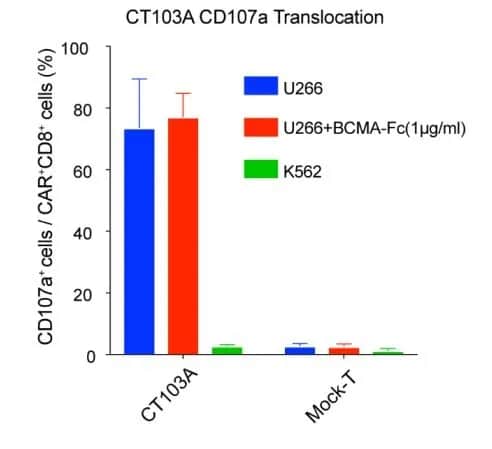
Image 2: The degranulation activity of CT103A was not inhibited by the addition of BCMA-Fc. (CD107a is a sensitive indicator of cytotoxic function)
The phase 2 FUMANBA-1 study (NCT05066646) in the Chinese population demonstrated deep and durable responses with equecabtagene autoleucel in RRMM patients, with a high complete response (CR) rate of 82.4% and a 12-month PFS rate of 85.5%. However, the correlation between baseline serum sBCMA levels and clinical outcomes after equecabtagene autoleucel treatment remains to be verified. This study aimed to explore the potential impact of baseline serum sBCMA levels on MM patients after equecabtagene autoleucel infusion.
Study Methods
Serum sBCMA levels were measured by enzyme-linked immunosorbent assay (ELISA). CAR transgene copy numbers in peripheral blood were monitored using digital droplet polymerase chain reaction (ddPCR). In this subanalysis, univariate and multivariate logistic regression models were used to evaluate baseline serum sBCMA levels and other characteristics potentially associated with achieving CR/sCR. Non-parametric tests and t-tests were used to analyze the differences in the impact of baseline sBCMA levels on the pharmacokinetics, pharmacodynamics, and efficacy of equecabtagene autoleucel treatment.
Study Results
The median baseline sBCMA level was 225.1 ng/mL in 91 RRMM patients without prior CAR-T therapy. Baseline sBCMA levels were not associated with age or gender but were significantly higher in patients with high tumor burden (defined as bone marrow plasma cells ≥50%; high tumor burden median: 605.9 ng/mL, low tumor burden median: 206.2 ng/mL; P=0.003), R-ISS stage III (R-ISS III median: 880.7 ng/mL, R-ISS I-II median: 213.2 ng/mL; P=0.012), DS stage III (DS III median: 409.1 ng/mL, DS I-II median: 130.5 ng/mL; P=0.0005), and BCMA expression ≥50% (BCMA ≥50% median: 360.0 ng/mL, BCMA expression <50% median: 127.9 ng/mL; P=0.012). Patients with baseline sBCMA levels ≥225.1 ng/mL were defined as the high sBCMA group, and those with baseline sBCMA levels <225.1 ng/mL were defined as the low sBCMA group. There were no significant differences between the high and low sBCMA groups in CAR-T cell expansion, AUC (Area Under the Curve) from 0-28 days, AUC from the start to the last time point, or cell persistence. Descriptive analysis showed that the baseline sBCMA levels were comparable between CR/sCR patients (median: 228.06 ng/mL) and non-CR/sCR patients (median: 185.13 ng/mL).
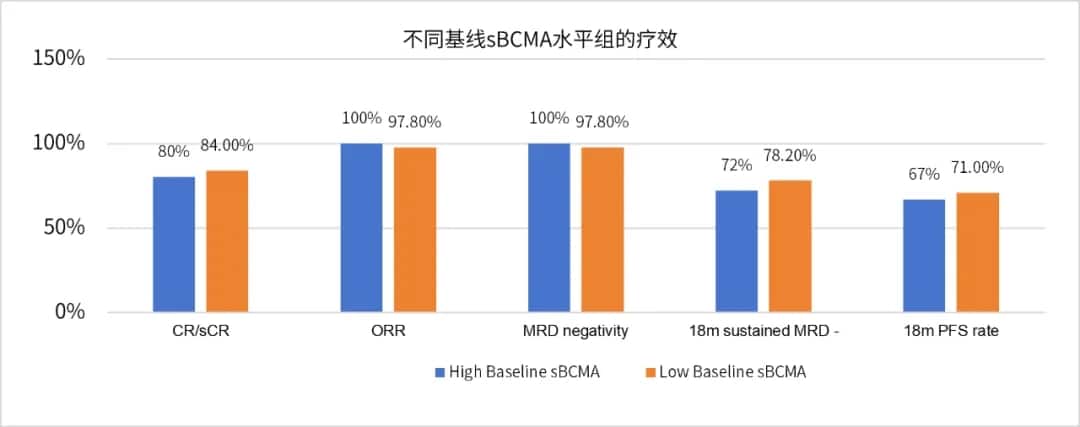
Image 3
Patients with high baseline serum sBCMA levels had an ORR of 100% and a ≥CR rate of 80%, while those with low baseline serum sBCMA levels had an ORR of 97.8% and a ≥CR rate of 84% (Image 3). Univariate analysis of baseline characteristics, including gender, age, extramedullary disease, high-risk cytogenetics, tumor burden, R-ISS disease staging, bridging therapy, and baseline sBCMA, showed no significant association with CR/sCR. There were no significant differences in MRD negativity rates, 18-month sustained MRD negativity rates, PFS, or OS between different baseline sBCMA level groups (Image 4).
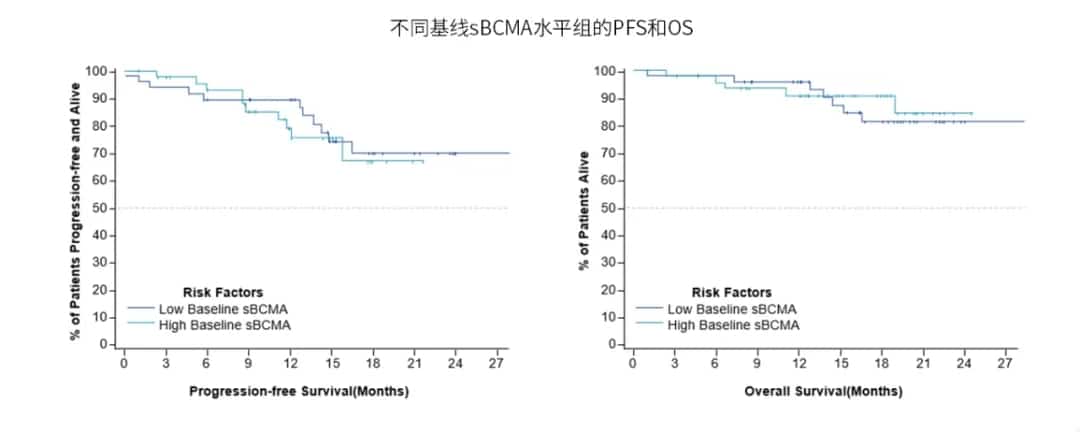
Image 4
Conclusion
In the FUMANBA-1 study, the median baseline sBCMA level was 225.1 ng/mL among 91 patients without prior CAR-T treatment history. Patients with high tumor burden, R-ISS stage III, DS stage III, and high BCMA expression had significantly higher sBCMA levels at baseline. However, the baseline sBCMA high-level group (sBCMA ≥ 225.1 ng/mL) and the baseline sBCMA low-level group (sBCMA < 225.1 ng/mL) showed comparable expansion and persistence of ijecabtagene autoleucel after infusion. There were no significant differences between the high and low baseline sBCMA groups in terms of overall response rate (ORR), complete response rate (CR), minimal residual disease (MRD) negativity rate in the overall population, 18-month sustained MRD negativity rate, and 18-month progression-free survival (PFS).

Figure 5
These results indicate that the efficacy of ijecabtagene autoleucel is not affected by baseline sBCMA levels, making it an ideal choice as a more universally applicable and promising treatment strategy for patients with relapsed/refractory multiple myeloma.
Based on the unique fast off-rate and low exhaustion advantages of the fully human CAR structure of ijecabtagene autoleucel, its dissociation kinetics are very similar to those of healthy T-cell receptors (TCRs). Even in a high sBCMA environment, ijecabtagene autoleucel can maintain continuous activation, cytotoxicity, and proliferation, with low self-exhaustion [3]. Therefore, regardless of the patient’s baseline sBCMA level before treatment, the potent and persistent anti-tumor activity of ijecabtagene autoleucel remains unchanged in vivo (Image 6). Studies have shown that the median persistence of ijecabtagene autoleucel in patients is 419 days, exceeding the critical in vivo persistence threshold of 280 days for CAR-T cells, which has been proven to significantly improve the overall survival (OS) rate of multiple myeloma patients, leading to survival benefits [4,5].
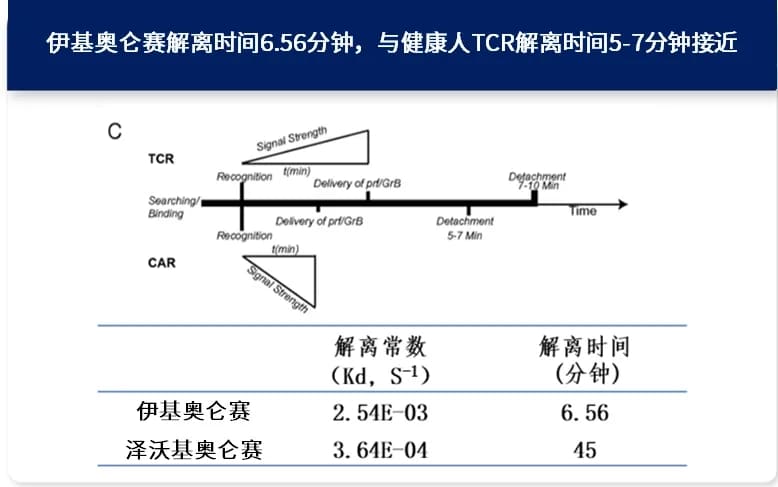
Image 6: The dissociation time of ijecabtagene autoleucel is 6.56 minutes, close to the dissociation time of 5-7 minutes for healthy TCRs. The fast off-rate is beneficial for reducing self-exhaustion, allowing long-term persistence and durable efficacy in patients [3].
The lead investigators of this clinical study, Professor Qiu Lugui from the Institute of Hematology and Blood Diseases, Chinese Academy of Medical Sciences, and Professor Li Chunrui from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, stated: “sBCMA level is not only an important biomarker of tumor burden in multiple myeloma patients but also a key factor affecting prognosis. Its accumulation in vivo can bind to BCMA on the surface of myeloma cells, thereby competitively inhibiting the binding of BCMA CAR-T cells. Relevant studies have reported that the efficacy of various BCMA CAR-T products, including Ide-cel (idecabtagene vicleucel), Cilta-cel (ciltacabtagene autoleucel), as well as BCMA-targeted bispecific antibodies, is affected by baseline sBCMA levels [6-13]. However, ijecabtagene autoleucel was carefully selected during its development and design, and its in vitro preclinical studies have already verified that its efficacy is not affected by sBCMA. The results of this study further confirm that ijecabtagene autoleucel can overcome the impact of baseline sBCMA, providing more significant and durable responses to patients regardless of their tumor burden or baseline sBCMA levels, making it an ideal choice for patients with RRMM.”
Reference Material
[1] ASH 2023 abstract 2009.
[2] Blood. 2021;137(21):2890-2901.
[3] Davenport, A. J., et al.” Proceedings of the National. Academy of Sciences 115, no. 9: E2068–76.
[4] Blood (2022) 140 (Supplement 1):4564-4565.
[5] Zhao, WH., et al. J Hematol Oncol 15, 86 (2022).
[6] Curr. Issues Mol. Biol. 2022, 44, 1463–1471.
[7] ASH 2023 abstract 2009.
[8] Pathol Oncol Res. 2023; 29: 1611171.
[9] ASH 2023 abstract 3345.
[10] Blood.2024 Mar 28;143(13):1211-1217.
[11] Transplant Cell Ther.2024 Mar 7:S2666-636(24)00253-7.
[12] Shah N, et al. ASH 2021, Blood 2021; 138:1739.
[13] Blood. 2021;137(21):2890-2901.
Reviewed by: Cherry
Layout: Cherry
Execution: Quinta
Content Source:医脉通血液科
