Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

The Rise of China’s CAR-T Therapy: New Hope for Multiple Myeloma Patients
**The Rise of China’s CAR-T Therapy: New Hope for Multiple Myeloma Patients**

Multiple Myeloma
#CARTTherapy #CART #MultipleMyeloma #BloodCancer #patientstory
China’s CAR-T cell therapy has emerged as a highly effective and proactive option for treating relapsed and refractory multiple myeloma, bringing new hope to patients facing limited treatment options. This innovative therapy has shown significant effectiveness in clinical practice, offering renewed optimism to both patients and doctors.
Recently, a team of hematology experts from Zhejiang Hospital visited a 72-year-old multiple myeloma patient who had just begun treatment with a domestically developed CAR-T therapy targeting BCMA. This patient, suffering from severe bone pain and difficulty walking, sought help from the Advanced Medicine In China team. Following a thorough consultation, the experts arranged for the patient’s hospitalization. After multiple rounds of traditional chemotherapy and an autologous stem cell transplant, the disease had unfortunately relapsed, prompting the medical team to recommend CAR-T therapy as an alternative.
As one of the first in China to implement CAR-T therapy for multiple myeloma, Zhejiang’s hematology team has accumulated significant clinical experience with this treatment. “The patient’s overall response to treatment has been very positive,” said one of the experts, noting that follow-up examinations showed stable results, confirming the therapy’s efficacy and safety.
Multiple myeloma is a malignant plasma cell disorder primarily affecting older adults; however, it has been seen with increasing frequency in younger patients, including some in their forties. While the disease remains incurable, recent advancements in new drugs and treatment options have greatly improved patient survival times and quality of life.
CAR-T therapy, or chimeric antigen receptor T-cell therapy, involves extracting a patient’s own T cells and genetically modifying them to “weaponize” them against cancer cells. Once modified, these T cells are expanded in number outside the body and reintroduced into the patient, where they can further proliferate and continually attack tumor cells. Given the wealth of experience and demonstrated effectiveness of CAR-T therapy in China, it has shown promise in delivering better quality and duration of survival for patients.
The development of CAR-T therapy in China has advanced rapidly, with two CAR-T products now approved for the treatment of multiple myeloma. Experts emphasize that patients should seek guidance from highly experienced medical professionals in selecting the most suitable CAR-T therapy to ensure optimal effectiveness and treatment safety.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#ChinaCART #CARTTherapy #CancerTreatment #Immunotherapy #MedicalInnovation #HopeForMyeloma #CancerResearch #AdvancedMedicineChina #CancerCare
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

New Advances in Chinese CAR-T Cell Therapy: CD27 Armored BCMA CAR-T Shows Potential in Treating Relapsed Multiple Myeloma
**New Advances in Chinese CAR-T Cell Therapy: CD27 Armored BCMA CAR-T Shows Potential in Treating Relapsed Multiple Myeloma**

Multiple Myeloma
#BCMA #CD27#CARTCellTherapy #RRMM #MM #MultipleMyeloma #CART
Relapsed and refractory multiple myeloma (RRMM) is a challenging blood cancer that poses significant obstacles for patients. Although several BCMA (B-cell maturation antigen) targeting CAR-T cell therapies have been developed, achieving long-term remission and extended survival remains difficult. The recent introduction of CD27-armored BCMA CAR-T—CBG-002—by a Chinese medical team brings new hope for RRMM patients. Based on preclinical data, this therapy shows promising anti-myeloma activity, with Phase I clinical trial results further validating its efficacy and safety.
Research Highlights
CBG-002 is an innovative therapy that enhances traditional BCMA CAR-T by adding a CD27 costimulatory domain. The inclusion of CD27 improves CAR-T cell persistence and activity, contributing to stronger anti-cancer effects. Recently published Phase I research in Cancer Immunology Research indicates that this CD27-armored BCMA CAR-T therapy is not only well-tolerated but also shows notable efficacy in RRMM patients.
The study enrolled 11 RRMM patients, all of whom had undergone three or more prior treatments, with a median age of 54. The results showed that 81.8% of patients experienced only mild cytokine release syndrome (CRS) and no severe neurotoxicity. Even at a low dosage level (1-3×10^6 CAR-T cells/kg), CBG-002 achieved a high overall response rate (ORR) of 81.8%, with 45.5% of patients reaching stringent complete remission.
Future Outlook
The Phase I study of CBG-002 not only confirmed its efficacy but also demonstrated advantages in production time and cost. Compared to current products on the market, CBG-002 has a shortened production cycle of just 10 days, making it a timely and affordable treatment option for more patients. Amid global progress in CAR-T cell therapies, the innovation by this Chinese medical team positions it as a significant force in advancing cancer research worldwide.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CancerResearch #Immunotherapy #ChinaBiotech #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

CAR-T Cell Therapy as a New Option for Treating Multiple Myeloma
**CAR-T Cell Therapy as a New Option for Treating Multiple Myeloma**

Multiple Myeloma
“CAR-T cell therapy has now become a new option for treating patients with relapsed and refractory multiple myeloma, with hopes of changing the difficult situation in myeloma treatment where treatments are either ineffective or unavailable,” said Jin Jie, Director of the Hematology Department at the First Affiliated Hospital of Zhejiang University School of Medicine, in an interview with People’s Daily Health on October 23.
Early on October 23, Jin Jie visited the hospital ward to see a 72-year-old patient with multiple myeloma. Just the day before, the patient had begun receiving treatment with a domestically produced BCMA-targeting CAR-T drug.
“This 72-year-old patient came to the hospital due to bone pain and was even unable to walk steadily,” Jin recalled. After being diagnosed with multiple myeloma, the patient initially received a series of chemotherapy regimens that showed some efficacy. However, a year after undergoing autologous hematopoietic stem cell transplantation, the disease relapsed, and various subsequent treatments proved suboptimal. As a result, the patient opted for CAR-T therapy.
Since the approval of Equecabtagene Autoleucel last year, Professor Jin Jie became one of the first doctors nationwide to prescribe CAR-T drugs for multiple myeloma. This patient is her tenth multiple myeloma patient to receive this innovative therapy. Discussing the treatment outcomes, Jin noted, “The overall efficacy for patients has been very good.” A month ago, another patient who underwent this CAR-T therapy returned for a follow-up. “Everything was going well; we will continue to monitor the patient’s indicators until all data are stable.”
Multiple myeloma is a malignant plasma cell disease that predominantly affects older adults. Jin observed that the incidence of multiple myeloma has been trending younger in recent years, with patients in their forties commonly seen in clinical settings. Currently, multiple myeloma remains an incurable disease, and most patients face inevitable relapse. However, thanks to the emergence of new drugs and advances in treatment, both the efficacy and overall survival of patients have significantly improved in recent years.
“CAR-T therapy involves collecting a patient’s T cells and equipping them with a ‘weapon.’ These T cells are then expanded outside the body and re-infused into the patient, where they target and attack tumor cells, while also being able to proliferate in the patient’s body to eliminate cancer cells,” Jin explained. CAR-T therapy has the potential to give patients a higher quality of life.
CAR-T cell therapy has been advancing rapidly in the field of multiple myeloma, with multiple products already approved and available. In Jin’s view, patients should, under professional guidance, choose drugs and treatments with extensive clinical experience and proven efficacy.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
**#MultipleMyeloma #CAR_TCellTherapy #CancerTreatment #Immunotherapy #Hematology #CellTherapy #MedicalInnovation #PatientCare #Oncology #ZhejiangUniversity**
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s CAR-T Therapy as a Beacon of Hope: A New Light for Multiple Myeloma Patients
**China’s CAR-T Therapy as a Beacon of Hope: A New Light for Multiple Myeloma Patients**

Multiple Myeloma
#CAR-TTherapy #MultipleMyeloma #PatientStory #CancerSurvivor
In recent years, CAR-T cell therapy in China has spearheaded a revolution in hematologic cancer treatment. This innovative immunotherapy has brought a glimmer of hope to many patients, especially those with relapsed and refractory cases who have exhausted traditional treatments. We recently interviewed Ms. Chen, a 53-year-old multiple myeloma patient who reached complete remission (CR) following CAR-T therapy, in hopes that her journey will inspire confidence and encouragement in other patients facing similar challenges.
**Ms. Chen’s Treatment Journey**
Ms. Chen, once a restaurant owner, was misdiagnosed with lumbar spondylolisthesis in 2019 due to chronic back pain and underwent surgery. However, her symptoms worsened post-surgery, and she was ultimately diagnosed with multiple myeloma with high-risk cytogenetic features. The sudden diagnosis was devastating, and she considered abandoning treatment. Encouraged by her family and reassured by her doctors, she decided to face the disease head-on and began treatment.
After multiple rounds of chemotherapy, Ms. Chen saw some improvement, but her condition continued to relapse. As her condition worsened and traditional therapies proved ineffective, she reached out to our Advanced Medicine in China team. After an expert consultation, Ms. Chen was offered the chance to receive CAR-T therapy, and with the support of her family and medical team, she found renewed hope.
**The Miracle of CAR-T Therapy**
A few days after her cells were collected and reinfused, Ms. Chen experienced side effects such as fever and headache. With close monitoring by our expert team, her side effects gradually came under control. After completing CAR-T therapy, her condition was effectively managed, allowing her to return to a normal lifestyle. Today, she can handle household chores and has gradually returned to work, leading a healthy and fulfilling life.
**Ms. Chen’s Advice and Encouragement**
-
**Trust Science and Doctors**: Persist with treatment and believe in the power of science—don’t give up easily.
-
**Maintain Good Communication**: Work closely with your doctors and trust their expertise.
-
**Stay Positive**: Approach the illness with positivity, avoiding anxiety and fear.
-
**Focus on Nutrition**: Ms. Chen believes dietary supplementation is better than medication and boosts immunity through balanced nutrition.
-
**Embrace a Healthy Lifestyle**: She often walks in the park, enjoys sunlight, and cherishes a restored, healthy life.
**Expert Opinion: The Advantages and Prospects of China’s CAR-T Therapy**
CAR-T therapy has become a groundbreaking treatment option due to its high specificity for hematologic malignancies. Compared with traditional therapies, China’s CAR-T therapy offers distinct advantages:
-
**High Specificity**: Chinese CAR-T cells can precisely identify and destroy cancer cells, reducing harm to normal tissues.
-
**Short Treatment Cycle**: Unlike conventional multi-round treatments, China’s CAR-T therapy typically requires only one infusion, with fewer side effects.
-
**Deep and Durable Responses**: Chinese CAR-T cells can survive in the body long-term and continually eliminate cancer cells.
Data shows that the overall survival rate for multiple myeloma patients treated with CAR-T is 92.9% over three years, highlighting its tremendous potential in enhancing both patient quality of life and longevity.
**Conclusion**
China’s CAR-T therapy brings new hope to patients with relapsed and refractory hematologic malignancies, and cases like Ms. Chen’s are on the rise. We hope her story will offer strength and confidence to patients currently battling illness.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #MedicalBreakthrough #ChinaMedicalAdvances #Immunotherapy #HealthcareInnovation #HopeAndHealing #AdvancedMedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

What Kind of Multiple Myeloma Patients Are Suitable for CAR-T Therapy in China?
# What Kind of Multiple Myeloma Patients Are Suitable for CAR-T Therapy in China?

Multiple Myeloma
#MultipleMyeloma #CAR_Therapy #MM #CART #RRMM #Hematology
**Multiple Myeloma** (MM) is a malignant hematological disease that affects plasma cells. In recent years, various drugs and treatment methods, such as proteasome inhibitors, immunomodulatory drugs, and autologous stem cell transplantation, have significantly improved the prognosis of multiple myeloma patients. However, a considerable number of patients still experience relapses and refractory disease. For these patients, CAR-T cell therapy is emerging as a groundbreaking and effective treatment option.
China has made remarkable progress in CAR-T therapy technology in recent years, becoming a global leader in the field of hematological diseases. This has attracted patients worldwide to seek this advanced treatment. So, which multiple myeloma patients are suitable to receive CAR-T treatment in China?
## 1. **Indications: Preferred Choice for Drug-Resistant/Relapsed/Refractory Multiple Myeloma Patients**
CAR-T therapy is typically recommended for multiple myeloma patients who have developed resistance to standard treatments (such as proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies) and are in their third line of treatment or beyond. In China, CAR-T therapy has broader indications and better efficacy.
### Suitable Patient Types:
-
Relapsed or refractory multiple myeloma patients, typically those who have undergone at least second-line treatments.
-
Patients with high-risk genetic characteristics, such as certain mutations (e.g., 17p deletion, t(4;14) translocation).
-
Patients unable to tolerate traditional treatments, including chemotherapy, other targeted therapies, stem cell transplantation, or immunomodulatory treatments, due to poor tolerance or severe side effects.
-
Patients who are BCMA-positive.
-
Patients whose disease continues to progress despite other treatments, especially chemotherapy.
-
Patients who are ineligible for stem cell transplantation.
-
Patients with severe symptoms not responsive to conventional treatments, such as bone pain, anemia, hypercalcemia, and kidney damage.
-
High tumor burden patients—experts generally reduce the tumor burden first before administering CAR-T therapy, often through bridging or sequential treatments.
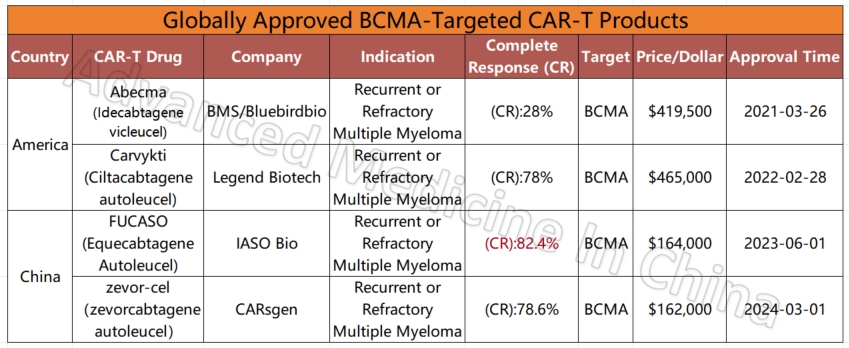
Among the four global CAR-T products targeting the BCMA marker in multiple myeloma, two Chinese products stand out for their efficacy and affordability. The most effective product currently is IASO Bio’s FUCASO (Equecabtagene Autoleucel), with a complete response (CR) rate of 82.4%.
## 2. **Patients in Good Physical Condition**
CAR-T therapy is essentially a powerful immunotherapy. Although it has remarkable efficacy, it also carries some risks of side effects, including cytokine release syndrome (CRS) and neurotoxic reactions. China’s CAR-T treatment system is highly developed, with established treatment plans and consensus. The country has extensive experience in managing CRS, neurotoxins, and side effects, keeping the risks very low. Moreover, the expert doctors at Advanced Medicine in China have substantial experience in managing these risks. Nonetheless, patients are typically required to have stable physical health to withstand the risks associated with the treatment.
– Overall good physical function; patients with heart, lung, liver, or kidney dysfunction may require a secondary evaluation by an expert team.
– Patients need to score 0-1 on the ECOG performance status scale, indicating that they are capable of daily activities and self-care.
## 3. **Patients with Financial and Time Support for CAR-T Therapy**
CAR-T therapy’s high cost and complex manufacturing process require patients and their families to have financial support. CAR-T therapy in China is significantly more affordable than in Western countries. While CAR-T treatments in the U.S. may cost $600,000 to $700,000, China’s approved CAR-T treatments cost around $100,000, about one-fifth to one-seventh of U.S. prices. Nevertheless, it remains a high-cost treatment, so patients need to be fully aware of the expenses involved.
China has invested heavily in CAR-T research in recent years. Many top hospitals and research institutions conduct related clinical trials. China accounts for over 50% of global CAR-T clinical trials. If a patient qualifies for a clinical trial, participating in it could be a more economical option, offering access to the latest CAR-T therapies, sometimes costing only tens of thousands of dollars or even free.
The CAR-T treatment process is relatively long. It involves collecting T cells, modifying them, re-injecting them, and close monitoring, requiring the patient to have enough time and patience to complete the entire process. The fastest known case took two weeks to complete all steps and discharge with complete response (CR), but generally, the process takes about four weeks or longer, depending on the patient’s condition.
For patients who can afford the treatment and have the time to complete it, CAR-T therapy in China is undoubtedly an attractive option.
## Conclusion
China’s leading position in CAR-T cell therapy technology and lower treatment costs make it a popular destination for multiple myeloma patients worldwide. It offers new hope for relapsed or refractory multiple myeloma patients. By working with experienced medical teams, patients can receive more personalized treatment plans and benefit from the rapid development of CAR-T therapy globally.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #ChinaMedicalInnovation #Immunotherapy #MyelomaTreatment #AdvancedMedicine #RelapsedMyeloma #CancerResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Recommended Myeloma Expert – Professor Qiu Luguo – A Leading Specialist in Hematology and Multiple Myeloma
### Recommended Myeloma Expert – Professor Qiu Luguo – A Leading Specialist in Hematology and Multiple Myeloma

Expert
We at Advanced Medicine In China are proud to have Professor Qiu Luguo as our contracted expert. He provides telemedicine consultations for our international patients and can arrange for them to travel to China through a special fast-track system. Patients can receive treatment in the hospital where Professor Qiu works, under his direct supervision as their attending physician, ensuring comprehensive and professional care throughout the treatment process.
Professor Qiu is renowned in the field of hematologic oncology, particularly for treating patients who have not responded well to standard therapies. His deep expertise and innovative treatment approaches have brought new hope to patients, especially those with complex conditions. Through our services, international patients can not only access cutting-edge treatment recommendations via telemedicine but also come to China to receive the most advanced medical care.
The process is straightforward and efficient. Patients only need to submit the following documents, and our team will assist in arranging everything from teleconsultation to full treatment in China:
-
Pathology report
-
Latest discharge summary
-
Detailed description of the condition
-
History of treatment and its effectiveness
We recommend Professor Qiu for his exceptional achievements and extensive experience, which have provided a wide range of treatment options for countless hematologic cancer patients. His international reputation further affirms his outstanding contributions to the fields of hematology and multiple myeloma.
### Expert Profile
Professor Qiu Luguo is the Director of the Lymphoma Diagnosis and Treatment Center at the Hematology Hospital, Chinese Academy of Medical Sciences, and Director of the Tianjin Cord Blood Hematopoietic Stem Cell Bank. He has long been dedicated to both clinical and basic research in hematologic diseases. Professor Qiu is a recipient of the State Council’s special government allowance and has been recognized as an Outstanding Young and Middle-aged Expert by the National Health Commission of China. As a leading authority in lymphoma and multiple myeloma treatment, he is also a member of several international academic organizations, including the International Myeloma Society (IMS) and the International Myeloma Working Group (IMWG).
### Areas of Expertise
Professor Qiu is highly skilled in the diagnosis and treatment of lymphatic system tumors, particularly in the precise diagnosis, stratified treatment, and prognosis evaluation of lymphoma, chronic lymphocytic leukemia, and multiple myeloma. He has also made significant progress in the application of hematopoietic stem cell transplantation technology, offering new treatment options for patients with hematologic diseases.
### Research Contributions and International Recognition
Throughout his 30-plus-year career, Professor Qiu has completed more than 30 national research projects and published nearly 700 papers in leading academic journals, with over 160 of them in SCI-indexed journals. His research achievements have garnered widespread attention in China and have been highly regarded in the international hematology community. He has been invited to speak at numerous international conferences and actively promotes global academic collaboration and exchange.
### Major Research Directions
Professor Qiu’s research focuses on the pathogenesis and precision treatment strategies for lymphatic system tumors. His work spans a broad range of fields, from basic research to clinical applications, covering the diagnosis, prognosis, and personalized treatment of lymphoma, multiple myeloma, and chronic lymphocytic leukemia. Additionally, he has conducted in-depth studies on hematopoietic stem cell transplantation and stem cell engineering, striving to develop innovative therapies that improve patient cure rates.
### Publications and Scientific Achievements
Professor Qiu has authored five major medical books, holds five national invention patents, and has received multiple first-place awards for his scientific achievements in China. His groundbreaking work in the genomics of multiple myeloma and chronic lymphocytic leukemia has made significant progress in early diagnosis and targeted treatment of these diseases. His research has not only advanced the treatment of lymphatic system tumors but also set an example for young researchers in the field of hematology.
With his exceptional clinical and research accomplishments, Professor Qiu Luguo has become a key figure in the global hematology community. His contributions have not only brought hope to patients but also laid a solid foundation for future advancements in the treatment of blood diseases. Some of his major publications include:…
-
Genomic and transcriptomic profiling reveals distinct molecular subsets associated with outcomes in mantle cell lymphoma. J Clin Invest. 2022 Feb 1;132(3):e153283. (IF: 19.456)
-
Indirubin-3′-monoxime acts as proteasome inhibitor: Therapeutic application in multiple myeloma. E Bio Medicine. 2022 Apr;78:103950. (IF:11.205)
-
A Phase II Trial of the Bruton Tyrosine-Kinase Inhibitor Zanubrutinib (BGB-3111) in Patients with Relapsed/Refractory Waldenström Macroglobulinemia. Clin Cancer Res. 2021 Oct 15;27(20):5492-5501. (IF: 12.531)
-
A Phase I Study of a Novel Fully Human BCMA-Targeting CAR (CT103A) in Patients with Relapsed/Refractory Multiple Myeloma. Blood, 2021 May 27;137(21):2890-2901. (IF: 22.133)
-
High incidence of MYD88 and KMT2D mutations in Chinese with chronic lymphocytic leukemia. Leukemia. 2021 Aug;35(8):2412-2415. Jan 22. (IF:12.883)
-
YWHAE/14-3-3ε expression impacts the protein load contributing to proteasome inhibitor sensitivity in multiple myeloma. Blood. 2020,136(4):468-479. (IF:16.509)
-
Monitoring the cytogenetic architecture of minimal residual plasma cells indicates therapy-induced clonal selection in multiple myeloma. Leukemia. 2020,34(2):578-588. (IF:11.528)
-
Destabilizing NEK2 overcomes resistance to proteasome inhibition in multiple myeloma. J Clin Invest. 2018,128(7):2877-2893. (IF:12.87)
-
Epigenetic silencing of miR-137 induces drug resistance and chromosomal instability by targeting AURKA in multiple myeloma. Leukemia. 2017.31(5):123-1135. (IF:11.412)
-
Intratumoral genetic heterogeneity and number of cytogenetic aberrations provide additional prognostic significance in chronic lymphocytic leukemia. Genet Med.19(2):182-191. (IF:7.756)
### Online Consultation and Appointment Information
Professor Ke currently offers online video consultation services. Patients can make appointments through the following methods:
WhatsApp: Https://wa.me/+8613717959070
Wechat:doctors0152
Email: doctor.huang@globecancer.com
#HematologyExpert #MultipleMyeloma #LymphomaTreatment #BloodCancer #StemCellTransplantation #MedicalInnovation #Telemedicine #InternationalHealthcare #AdvancedMedicineInChina #PrecisionMedicine #ProfessorQiuLuguo #CancerResearch #GlobalHealth #PatientCare #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Why Are More and More International Patients Coming to China for CAR-T Cell Therapy?
Why Are More and More International Patients Coming to China for CAR-T Cell Therapy?

CARTtherapy
#CARTtherapy #InternationalPatient #CART #Multiplemyeloma #lymphoma #leukemia
In recent years, CAR-T cell therapy, as an innovative cancer treatment, has rapidly gained widespread attention globally. China, as a leading country in the CAR-T treatment field, has attracted an increasing number of patients from Europe and Asia due to its advanced technology, high-quality medical resources, and relatively lower treatment costs. So, why are more international patients choosing to come to China for CAR-T treatment? Here are some key reasons worth noting:
### 1. **Advanced CAR-T Treatment Technology**
Over the past decade, China has made tremendous strides in medical research, particularly in the field of cancer immunotherapy. Not only has China successfully developed various CAR-T therapies, but it has also built a comprehensive treatment protocol and safety monitoring system to ensure efficient and safe treatment for patients. Additionally, China has accumulated extensive experience in managing treatment-related side effects, such as CRS (Cytokine Release Syndrome) and neurotoxicity.
Currently, more than 50% of the world’s clinical trials for CAR-T therapy are conducted in China. Of the 11 CAR-T products already on the global market, five are from China. These include products for multiple myeloma (BCMA CAR-T), diffuse large B-cell lymphoma (CD19 CAR-T), and leukemia. Compared to similar products in the U.S. for multiple myeloma, China’s CAR-T products are not only fully humanized but also boast the highest complete remission rates among all available products, reaching 82.4%. The CR data for China’s lymphoma and leukemia CAR-T products are also impressive, at 77.6% and 82.1%, respectively.
### 2. **International Certification and Clinical Trial Opportunities**
Several hospitals and research institutions in China have gained international recognition for their CAR-T therapies. Patients can choose between commercial CAR-T treatments or participate in clinical trials. For some CAR-T treatments that have not yet been approved or are hard to access in Europe or Asia, patients can receive these cutting-edge therapies in China earlier.
Just a few days ago, Chinese scholars’ research on universal CAR-T cell therapy was featured on the cover of *Nature*. Various other studies on Chinese CAR-T therapies are frequently published in major medical journals. For example, a Chinese medical team published a study on dual-target CAR-T in *JAMA Oncology*, showing a 100% stringent complete remission (sCR) rate in high-risk multiple myeloma patients. Additionally, a research team from Zhejiang, China, published clinical data in *Cell Discovery*, highlighting the breakthrough of the fourth-generation anti-CD19 CAR-T cell therapy in treating relapsed/refractory large B-cell lymphoma with exceptional anti-tumor capabilities.
### 3. **Relatively Low Treatment Costs**
Compared to the million-dollar treatment costs in Western countries, CAR-T treatment in China is relatively affordable, often costing just 1/5 to 1/7 of the price in the U.S. For instance, the cheapest CAR-T product for multiple myeloma in the U.S. costs $420,000, and when including hospitalization and other expenses, the total cost can reach around $700,000. In contrast, similar Chinese products, with equal or even better effectiveness, cost only $160,000, and hospitalization and other fees are much lower, often under $10,000. Some treatment options are available for just a few tens of thousands of dollars, and in certain cases, treatment is even completely free.
Patients from Europe and Asia can receive world-class CAR-T therapy in China at a much lower cost, providing an affordable option for those who find the high costs of treatment elsewhere prohibitive. Moreover, a range of one-stop medical services, including hospital visits, translation assistance, transportation, accommodation, and even family care, can significantly enhance the patient experience for international patients.
### 4. **World-Class Expert Teams**
China is home to a group of internationally renowned oncology and immunotherapy experts who have extensive clinical experience and academic achievements in hematological cancers and CAR-T therapy. Patients receive personalized treatment plans designed by leading experts, ensuring that each treatment is precisely tailored to their specific condition, maximizing effectiveness and minimizing risks.
For example, Professor Huang Xiaojun, Director of the Institute of Hematology at Peking University, is one of the world’s pioneers in haploidentical transplantation, having developed China’s haploidentical hematopoietic stem cell transplantation model. His innovative approach has increased the 3-year survival rate of leukemia patients receiving haploidentical transplants from around 20% to approximately 70%, earning him the Distinguished Service Award from the Center for International Blood and Marrow Transplant Research.
### 5. **Efficient and Convenient Medical Services**
China has implemented a 144-hour visa-free transit policy for citizens of 54 countries and mutual visa exemptions with 24 countries. This is a major benefit for international patients seeking treatment in China. Moreover, China’s healthcare system is known for its efficiency and convenience, especially in treating major diseases. Appointment wait times are short, and there is minimal delay in receiving treatment. A “super green channel” is also available for international patients, ensuring quick diagnosis and treatment planning. From initial consultation to online meetings with specialists, the process typically takes less than a week, with hospital admission and treatment beginning within another week.
Compared to the long waiting periods in other countries, China’s efficient medical services significantly improve treatment success rates and patient satisfaction. CAR-T therapy, in particular, is known for its high efficiency. There was an international patient who went from initial consultation to complete remission and a safe return home in less than a month—a remarkable achievement considering CAR-T is a “living” drug that must be custom-prepared using the patient’s own blood cells.
### 6. **Abundant Success Stories**
Many international patients who have received CAR-T therapy in China have achieved remarkable results, with their cancers being effectively controlled or even cured. These success stories not only boost confidence among patients worldwide but also attract more people to China for treatment. By sharing these stories, more patients learn about China’s advantages in CAR-T therapy and choose it as their treatment destination.
For instance, Ethan, a patient from Singapore, achieved complete remission just four weeks after CAR-T treatment and has since recovered very well. There is also the story of a Russian patient with high-risk, late-stage multiple myeloma who received efficient treatment in China at a much more reasonable cost and with a faster treatment process.
### 7. **Conclusion**
In conclusion, as China continues to enhance its technological capabilities in CAR-T cell therapy, more and more patients from around the world are choosing to come to China for this cutting-edge cancer treatment. With its advanced medical technology, international services, and relatively lower treatment costs, China is becoming an important destination for cancer patients seeking treatment. If you or a loved one is searching for innovative cancer treatment options, consider CAR-T therapy in China for a chance at recovery!
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can contact us for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CellTherapy #CancerTreatment #Immunotherapy #HealthcareInnovation #MedicalTourism #ChinaHealthcare #CancerSurvivor #PatientStories #MedicalAdvancements #AffordableHealthcare #PersonalizedMedicine #GlobalHealth #HealthJourney #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
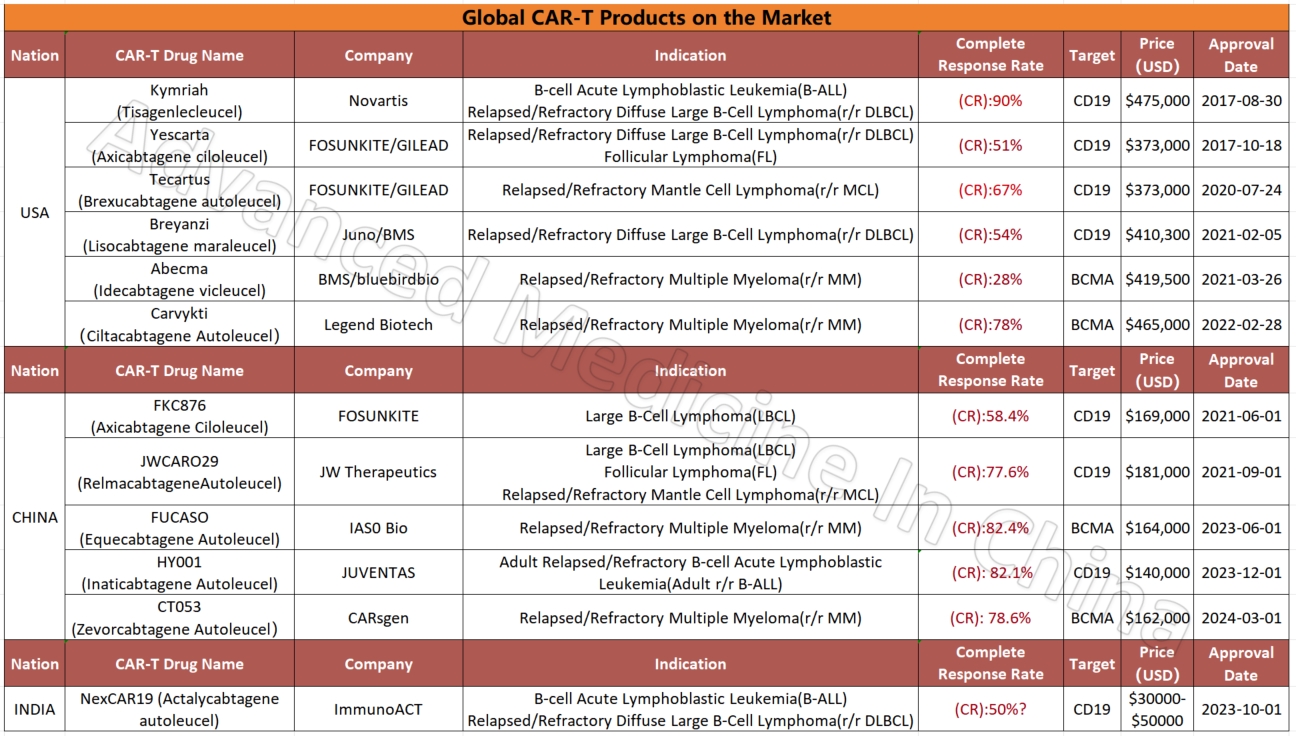
Global CAR-T Products on the Market and BCMA-Targeted CAR-T Products
Global CAR-T Products on the Market

CAR-T
Globally Approved BCMA-Targeted CAR-T Products
– Multiple Myeloma
Multiple Myeloma

 To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Chinese Medical Team Releases Dual-Target CAR-T Data: 100% Complete Remission Rate in High-Risk MM Patients**
**Chinese Medical Team Releases Dual-Target CAR-T Data: 100% Complete Remission Rate in High-Risk MM Patients**

High-Risk MM
#CARTTherapy #MultipleMyeloma #BCMA #CD19 #JAMAOncology #MM #HRMM #DualTarget
Once again, a Chinese medical team is at the forefront of innovation, making significant strides in the field of CAR-T cell therapy. A recent study published in *JAMA Oncology* (B-Cell Maturation Antigen/CD19 Dual-Targeting Immunotherapy in Newly Diagnosed Multiple Myeloma) revealed remarkable results for the dual-target BCMA/CD19 CAR-T therapy in high-risk newly diagnosed multiple myeloma (NDMM) patients. In this study, all 19 participants achieved stringent complete remission (sCR) and were found to be minimal residual disease (MRD) negative, resulting in an astounding 100% remission rate.
**CAR-T Therapy: A Breakthrough in Hematologic Cancer Treatment**
Multiple myeloma (MM) is a challenging hematologic cancer, particularly for patients with high-risk features, where traditional treatments often show limited efficacy. CAR-T cell therapy is an innovative immunotherapy that reprograms a patient’s own T cells to recognize and attack cancer cells. In recent years, this therapy has shown significant success in treating leukemia, lymphoma, and relapsed/refractory MM (RRMM). The latest study further demonstrates that dual-target CAR-T therapy, focusing on BCMA and CD19, can significantly improve the prognosis for high-risk MM patients.
**The Impressive Results of China’s Dual-Target CAR-T Therapy**
The CAR-T therapy used in this study, GC012F, targets both B-cell maturation antigen (BCMA) and CD19 surface antigens, showing powerful anti-tumor effects. Every patient in the study not only achieved complete remission after treatment but also maintained a long-term MRD-negative status, meaning that traces of cancer cells were nearly undetectable post-therapy.
More importantly, the study highlighted the swift effectiveness of the Chinese GC012F therapy—patients reached their first complete remission in a median time of just 84 days, with MRD-negative status achieved in as little as 28 days. This rapid anti-tumor response provides a crucial treatment window for patients, significantly improving their prognosis.
**Safety and Future Prospects**
In addition to its impressive efficacy, GC012F demonstrated favorable safety. Only 27% of patients experienced mild to moderate cytokine release syndrome (CRS), and no cases of immune effector cell-associated neurotoxicity syndrome (ICANS) were observed. This is in stark contrast to previous studies in relapsed/refractory patients, where CRS rates were higher, highlighting the safety advantage of this therapy in newly diagnosed patients.
Though the study sample size was small, these findings bring new hope for high-risk multiple myeloma patients. As larger clinical trials are conducted and combination therapies are explored, this therapy has the potential to offer better survival outcomes for more patients.
**Conclusion**
China’s CAR-T cell therapy research continues to lead the way in hematologic cancer treatment. The success of the BCMA/CD19 dual-target CAR-T therapy not only demonstrates its strong efficacy in high-risk multiple myeloma but also provides a new direction for treating hematologic cancers globally. As research deepens, we anticipate that this groundbreaking therapy will bring new life-saving opportunities to more patients.
This breakthrough in Chinese CAR-T therapy represents not only scientific progress but also underscores China’s pivotal role in the global fight against cancer.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #Immunotherapy #MedicalInnovation #CancerResearch #ChinaHealthcare #HematologicCancer #CancerBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s BCMA CAR-T Therapy Offers Last Hope for Relapsed Multiple Myeloma Patients
### China’s BCMA CAR-T Therapy Offers Last Hope for Relapsed Multiple Myeloma Patients

CAR-T Therapy
Multiple myeloma remains a difficult-to-cure disease, and every year, countless patients around the world face this devastating diagnosis. While treatment options have improved in recent years, and patients’ survival times have extended, drug resistance remains a significant challenge. For patients who have experienced multiple relapses and are resistant to various treatments, the future can seem bleak. Fortunately, with the emergence of **BCMA CAR-T cell therapy**, a new ray of hope is illuminating the path for these patients.
This is the story we’re sharing today, about a 76-year-old female patient who regained control of her life through CAR-T therapy.
#### The First Encounter with the Disease
In 2020, this Chinese 76-year-old woman noticed a decline in appetite and a rapid weight loss, accompanied by persistent rib pain. She had no idea that these symptoms were early signs of multiple myeloma. After several medical tests, her Chinese doctors confirmed the diagnosis: 31% of her bone marrow cells were plasma cells, her M-protein levels reached 85.7 g/L, and calcium levels in her blood were abnormally high. Multiple bones throughout her body also showed signs of significant damage.
The diagnosis left the patient shocked and confused. Multiple myeloma wasn’t her first health challenge; she also had a history of Parkinson’s disease. However, she remained hopeful, believing that with the right treatment, her condition could be controlled. But fate had other plans.
#### The Struggle and Relapse
At the beginning of 2023, the patient’s condition relapsed rapidly. Due to a prolonged lack of regular treatment, her disease worsened, and she became resistant to many standard therapies. After extensive tests, her medical team discovered that she had a high-risk mSMART 3.0 profile, with genetic abnormalities including 1q21 amplification and a t(4;14) translocation, making her prognosis extremely poor. She was now considered a “triple-refractory” patient, resistant to three main treatment categories.
Faced with this complex and challenging situation, the Chinese doctors didn’t give up. After multiple discussions and research, they recommended a cutting-edge therapy — **BCMA CAR-T cell therapy**. This treatment involves modifying the patient’s immune cells to target and destroy cancer cells. However, to undergo this advanced therapy, the patient first needed to undergo **bridging treatment** to reduce the tumor burden in her body.
#### Bridging Treatment: A Turning Point
To prepare for CAR-T therapy, the Chinese doctors initially prescribed the **DKD regimen**, a combination therapy intended to quickly reduce the cancer cell load. However, the first treatment cycle didn’t bring significant improvement, and her free light chain levels remained high. Both the patient and her family felt disappointed, but the Chinese doctors acted quickly, switching to the **KAD regimen**, which included **liposomal doxorubicin** to further enhance the treatment’s effectiveness.
This adjustment brought immediate results. After a few weeks, her free light chain levels began to drop, and her condition showed partial remission (PR). This meant she was finally ready for CAR-T cell therapy.
#### The Miracle of CAR-T Therapy
CAR-T therapy is a breakthrough technology where the patient’s immune cells are extracted, genetically modified to recognize and destroy cancer cells, and then reintroduced into the body. These engineered cells act like precision-guided weapons, specifically targeting her cancer cells.
The patient responded positively to the treatment. Within just a few weeks, her condition was significantly controlled, with results showing a very good partial response (VGPR). For such a complex case of relapsed and refractory disease, this was a major success.
#### The Significance of the Story
This patient’s story highlights China’s latest advancements in the treatment of multiple myeloma. The use of **carfilzomib-based bridging therapy** paved the way for CAR-T therapy, successfully giving a patient with a poor prognosis a new chance at life. For those facing the dual challenges of drug resistance and relapse, **BCMA CAR-T therapy** offers not just a new treatment option, but a lifeline.
The product that has renewed hope for this 76-year-old Chinese woman is a BCMA-targeted CAR-T therapy that has already been launched in China. China is changing the fate of patients worldwide through advanced cancer treatments. Notably, in the field of CAR-T technology related to hematologic tumors and multiple myeloma, two BCMA CAR-T products have been approved in China. Especially commendable is Reindeer Bio’s independently developed fully human BCMA product, FUCASO, which has achieved an astonishing complete remission (CR) rate of 82.4%, the highest among all globally approved products. An increasing number of foreign multiple myeloma patients are now seeking treatment in China to access better therapies at more affordable prices. As China continues to develop cutting-edge medical therapies, aggressive treatments like CAR-T undoubtedly provide new hope for the future of multiple myeloma treatment.
Follow us for more stories of medical innovation and to witness how miracles of life are born in China!
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CancerTreatment #CAR_TTherapy #BCMACAR_T #CancerSurvivor #Immunotherapy #ChinaMedicalInnovation #CancerResearch #CancerHope #MedicalBreakthrough #MyelomaAwareness #AdvancedCancerCare #RelapsedMyeloma #LifeSavingTreatment #PatientSuccessStory #CancerCare #GlobalHealth #CAR_TSuccess #Oncology #CancerSupport
#ChineseMedical #ChinaHealthcare #MedicalCareInChina #HealthcareInChina · #USMedical #USHealthcare #HealthcareInUSA #AmericanMedical #MedicalCareInUSA #USAHealth #AmericanHealthcare
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Blood Cancer – Diagnosis and Treatment Tips
 Blood Cancer – Diagnosis and Treatment Tips
Blood Cancer – Diagnosis and Treatment Tips 

Blood Cancer
Blood cancer, encompassing types such as leukemia, lymphoma, and multiple myeloma, represents a significant health challenge in regions like Southeast Asia and India. These hematologic malignancies arise from blood-forming cells and can impact the bone marrow, blood, and various organs. The increasing prevalence of these diseases is concerning, yet advancements in diagnosis and treatment provide hope for patients.
## Understanding Hematologic Malignancies
 Hematologic malignancies include a variety of cancers such as:
Hematologic malignancies include a variety of cancers such as:
– **Leukemia**: Cancer of the bone marrow that leads to the overproduction of abnormal white blood cells.
– **Lymphoma**: Affects the lymphatic system and includes #Hodgkin and #nonHodgkinlymphoma.
– **Multiple Myeloma**: Cancer that forms in plasma cells, affecting the production of antibodies.
 The causes of these conditions are multifactorial, involving genetic factors, immune dysfunction, exposure to environmental toxins, and lifestyle choices. For instance, a diet high in processed foods, sedentary behavior, and high stress levels can contribute to an increased risk of developing these cancers.
The causes of these conditions are multifactorial, involving genetic factors, immune dysfunction, exposure to environmental toxins, and lifestyle choices. For instance, a diet high in processed foods, sedentary behavior, and high stress levels can contribute to an increased risk of developing these cancers.
## The Rising Incidence of Blood Cancer
The incidence of blood cancers is on the rise globally, particularly in countries with aging populations. In Southeast Asia and India, there has been a noticeable increase in cases of leukemia and lymphoma, necessitating better awareness and improved healthcare infrastructure.
## Diagnosis: Early Detection is Key
Early diagnosis is crucial for successful treatment outcomes. Patients experiencing symptoms such as unexplained fatigue, frequent infections, easy bruising, or swollen lymph nodes should seek medical advice promptly. Healthcare providers may utilize blood tests, bone marrow biopsies, and imaging studies to diagnose these conditions accurately.
## Treatment Advances: A Diverse Approach
Recent years have seen significant advancements in the treatment of hematologic malignancies, offering patients more options than ever before:
1. **Combination Chemotherapy**: Despite potential side effects, combination chemotherapy remains a cornerstone treatment. New regimens are being developed that combine traditional cytotoxic drugs with targeted therapies and immunotherapies to enhance efficacy and reduce toxicity.
2. **Targeted Therapies**: Drugs that specifically target cancer cell mutations or characteristics are revolutionizing treatment. For example, *Gleevec*, the first targeted therapy, has dramatically improved survival rates for chronic myeloid leukemia (#CML).
3. **Monoclonal Antibodies**: Often dubbed “biological missiles,” these treatments target specific proteins on cancer cells, allowing for more precise treatment with fewer side effects.
4. **Hematopoietic Stem Cell Transplantation**: This procedure remains one of the most effective treatments for certain types of blood cancer, especially when other treatments fail.
5. **Immunotherapy**: This approach harnesses the body’s immune system to fight cancer. Techniques like CAR-T cell therapy, which modifies a patient’s T cells to attack cancer cells, have shown promising results in treating various hematologic malignancies. China is at the forefront of CAR-T cell therapy research and application, particularly in treating hematologic malignancies. CAR-T therapy involves extracting a patient’s T cells, modifying them to specifically recognize and attack cancer cells. Clinical research and applications in China have shown remarkable effectiveness in treating leukemia, lymphomas, and multiple myeloma. Some studies indicate that patients receiving CAR-T treatment experience significantly higher long-term survival rates and lower recurrence rates. Furthermore, China has made significant progress in the standardization of CAR-T therapy, personalized treatment plans, and the development of technological platforms, enabling more patients to benefit from this innovative treatment.
## Hope for the Future
Countries like India and those in Southeast Asia are rapidly advancing in the field of blood cancer treatment. Collaborative efforts among healthcare professionals, research institutions, and patient advocacy groups are crucial for improving access to cutting-edge therapies. The development of treatment guidelines tailored to the regional context can ensure that patients receive the best possible care.
### Conclusion
Hematologic malignancies are challenging but treatable diseases. Through early diagnosis and the application of innovative therapies, patients can achieve significant improvements in their health and quality of life. As research continues to evolve, the prospect of long-term survival and even cures for blood cancers is becoming increasingly attainable. Empowering patients with knowledge and access to modern treatments is essential to fighting blood cancer effectively in #SoutheastAsia and #India.

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#HematologicMalignancies #LeukemiaAwareness #LymphomaResearch #MultipleMyeloma #BloodCancer #CancerResearch #CAR_Therapy #StemCellTransplant #Immunotherapy #TargetedTherapy #MonoclonalAntibodies #CancerTreatment #MedicalAdvancements #CancerSurvivor #HealthcareInnovation #ALL #AML
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**China’s CAR-T Therapy Brings Complete Remission for Primary Refractory Multiple Myeloma Patient**
**China’s CAR-T Therapy Brings Complete Remission for Primary Refractory Multiple Myeloma Patient**

Multiple Myeloma
#MultipleMyeloma #CARTtherapy #BloodCancer #CompleteRemission #CART
