2 years ago
2 years ago
Overview of Ciltacabtagene Autoleucel Approval
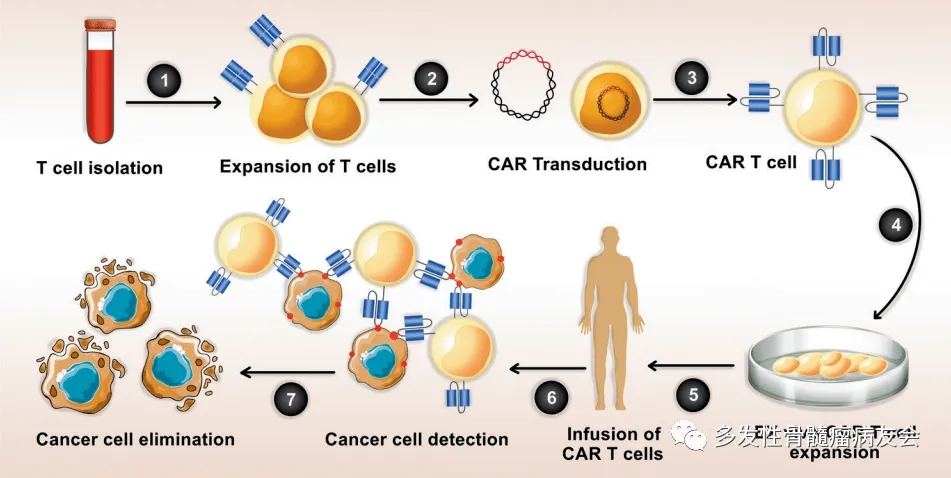
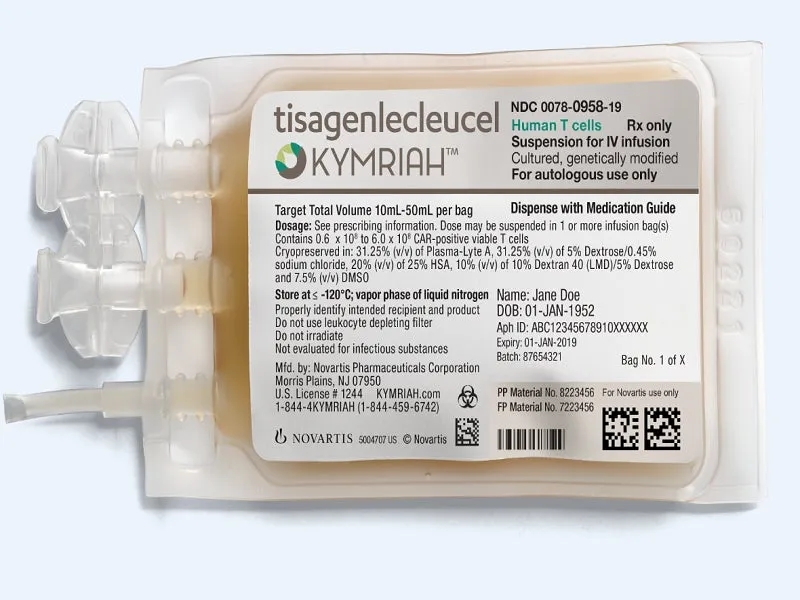
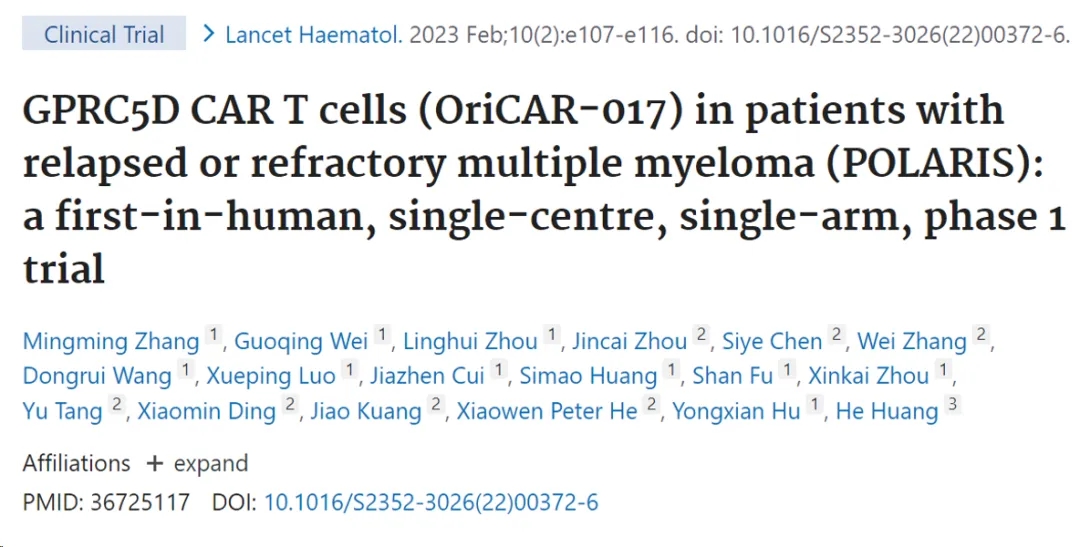
Overview of Ciltacabtagene Autoleucel Approval Ciltacabtagene Autoleucel: An Overview of Its Approval Journey From 2014 to 2022, it took Ciltacabtagene Autoleucel eight years to complete the journe...