 2 years ago
2 years ago
A Brief Analysis of Carvykti Multiple Myeloma
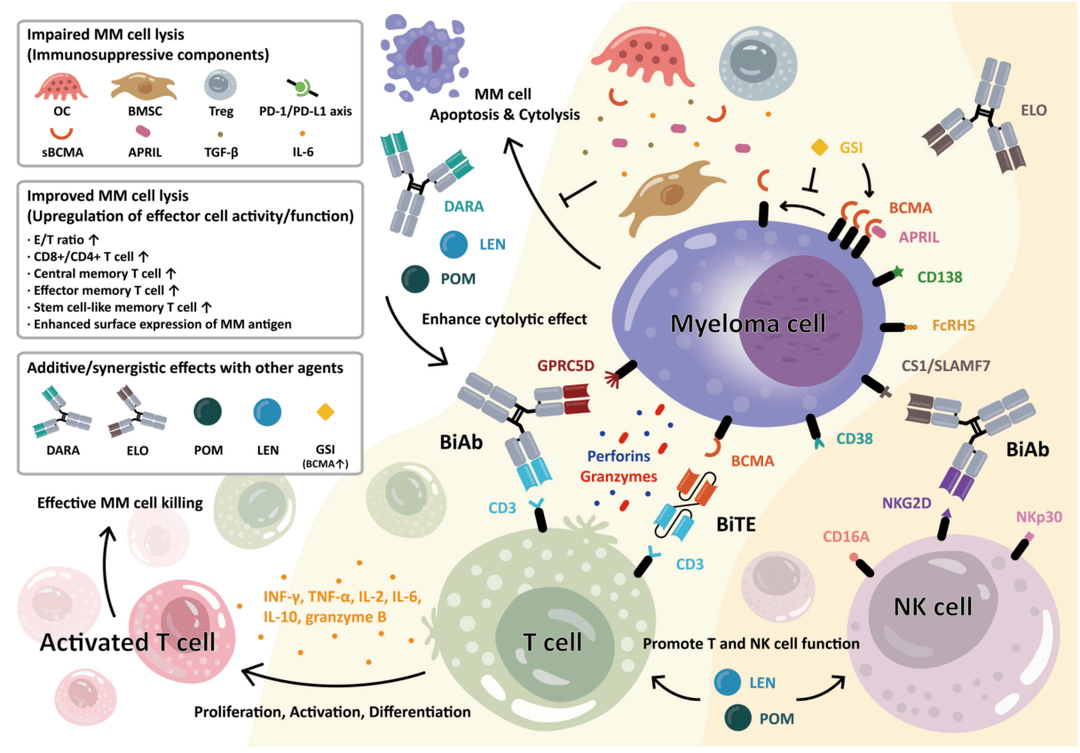
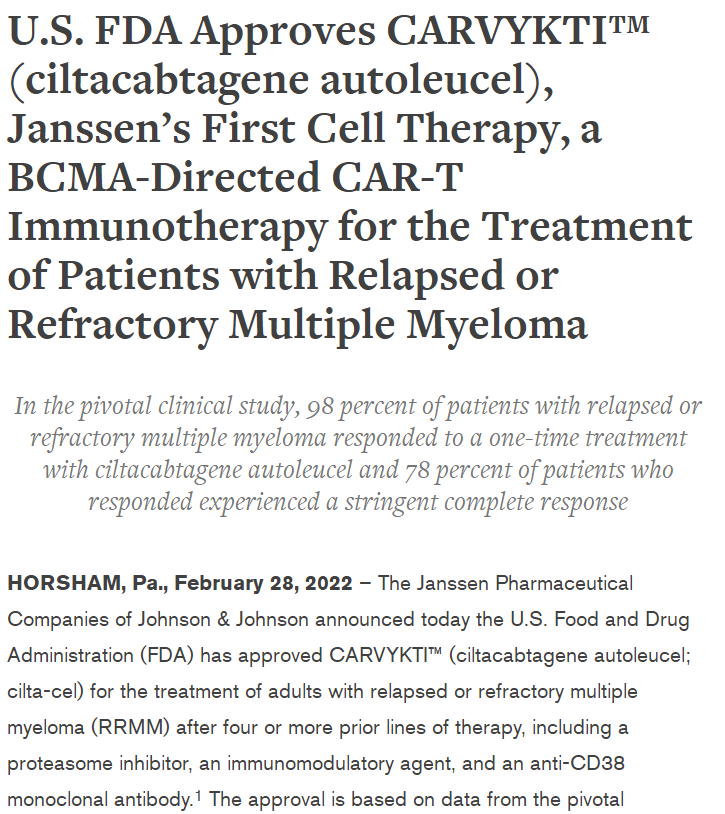
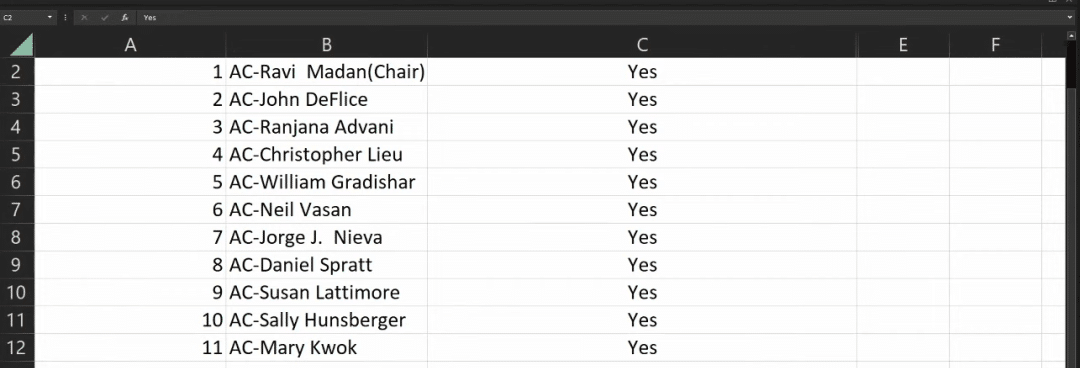
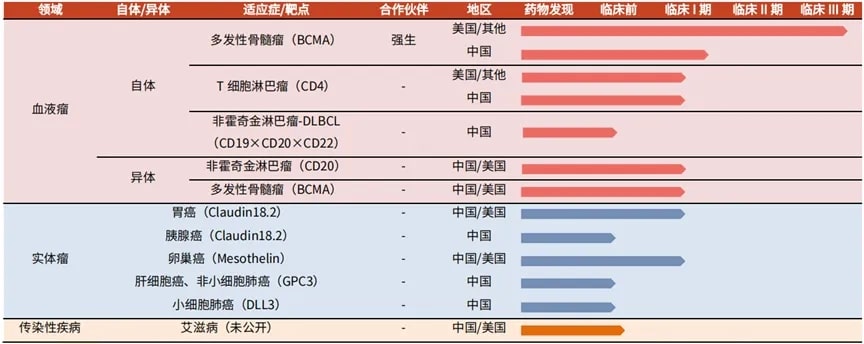
A Brief Analysis of Carvykti Multiple Myeloma Legend Biotech was founded in 2014 and is a multinational biopharmaceutical company focused on the research, development, and production of cell immuno...